ARTS trial evaluated aumolertinib as adjuvant therapy in patients with completely resected stage II–IIIB EGFR-mutated non-small-cell lung cancer, a population that remains at high risk of recurrence despite surgery and standard postoperative chemotherapy. The study was designed to determine whether prolonged treatment with a third-generation EGFR tyrosine-kinase inhibitor could further reduce postoperative relapse and improve disease control in this high-risk setting.
Title: Aumolertinib as adjuvant therapy in resected EGFR-mutated non-small-cell lung cancer (ARTS): a double-blind, multicentre, randomised, controlled, phase 3 trial
Authors: Liang Zhang, MD, Xiqin Zhang, BS, Lin Wu, MD, Wenqun Xing, BS, Chunling Liu, MS, Prof Peng Zhang, MD, Kai Chen, MD, Jianhua Shi, MS, Prof Shidong Xu, MD, Xiaodong Zhang, BS, Prof Xiaorong Dong, MD, Haohui Fang, BS, Xinmin Yu, BS, Yang Gao, MD, Prof Gaofeng Li, MHA, Zhenming Chen, MS, Shaonan Fan, MD, Xiaoqing Zhang, PhD, Ying Cheng, MDLiang Zhang, MD,
Xiqin Zhang, BS, Lin Wu, MD, Wenqun Xing, BS, Chunling Liu, MS, Prof Peng Zhang, MD, Kai Chen, MD, Jianhua Shi, MS, Prof Shidong Xu, MD, Xiaodong Zhang, BS, Prof Xiaorong Dong, MD, Haohui Fang, BS, Xinmin Yu, BS, Yang Gao, MD, Prof Gaofeng Li, MHA, Zhenming Chen, MS, Shaonan Fan, MD, Xiaoqing Zhang, PhD, Ying Cheng, MD
Published in Lancet Oncology, Feb 2026
Background
Patients with resected stage II–III non-small-cell lung cancer (NSCLC) remain at substantial risk of recurrence, particularly when tumors harbor sensitizing EGFR mutations (exon 19 deletion or L858R). While adjuvant chemotherapy offers only modest benefit, third-generation EGFR TKIs have reshaped the postoperative landscape, with a need for robust evidence in populations with high EGFR prevalence, including Chinese patients. The ARTS trial evaluated whether aumolertinib, a third-generation EGFR TKI, improves outcomes as adjuvant therapy following complete resection and standard postoperative treatment.
Methods and Study Design
ARTS was a double-blind, multicentre, randomized, placebo-controlled phase 3 trial conducted across 48 hospitals in mainland China. Adults (≥18 years) with stage II–IIIB (T3N2M0 allowed) non-squamous NSCLC and EGFR ex19del or L858R were eligible after complete resection and standard adjuvant therapy. Patients had ECOG 0–1 and no baseline brain metastases.
Participants were randomized 1:1 to receive aumolertinib 110 mg once daily or placebo, planned for 3 years, or until recurrence/discontinuation. Randomization was stratified by mutation type and stage. The primary endpoint was disease-free survival (DFS) in the modified intention-to-treat (mITT) population, assessed by blinded independent central review (BICR). Safety was evaluated in all patients receiving at least one dose.
Results
Between April 30, 2021, and May 17, 2022, 214 patients were randomized (107 per arm). Median age was 59 years (IQR 54–66); 56% were female, and 95% received prior adjuvant chemotherapy. The mITT population included 210 patients (106 aumolertinib; 104 placebo), after excluding a small number found to have stage I disease. At the April 15, 2024 cutoff, median follow-up was ~27.6 months in both arms.
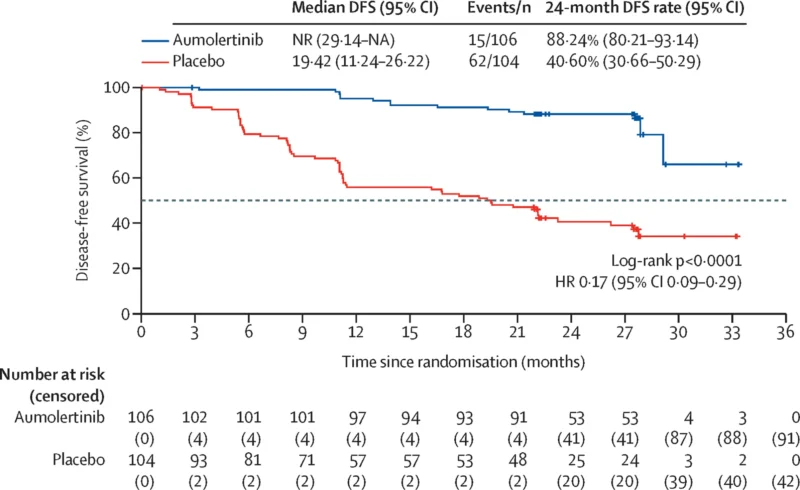
Aumolertinib produced a major DFS improvement versus placebo by BICR with HR 0.17 (95% CI 0.09–0.29; p<0.0001). Median DFS was not reached with aumolertinib, compared with 19.42 months (95% CI 11.24–26.22) with placebo. DFS curves separated early. Recurrence occurred in 14% (15/106) with aumolertinib versus 60% (62/104) with placebo.
At 2 years, BICR-assessed DFS rates were 88.24% (95% CI 80.21–93.14) with aumolertinib versus 40.60% (95% CI 30.66–50.29) with placebo. Distant metastasis was the most common recurrence pattern, occurring in 9% with aumolertinib versus 39% with placebo (BICR). Brain metastases were reported in 6% versus 16%, respectively, though these CNS findings require cautious interpretation due to limited event numbers and follow-up.
Overall survival (OS) was immature at cutoff, with deaths in 3% (3/106) vs 4% (4/104). Crossover occurred: 37% of control-arm patients with recurrence initiated aumolertinib per protocol criteria.
Treatment-emergent adverse events occurred in 98% of aumolertinib-treated patients versus 94% with placebo; grade 3–4 events were 27% vs 22%. Notable grade 3–4 events included increased creatine phosphokinase (7% vs 0%), QT prolongation (3% vs 3%), hypertension (1% vs 5%), and pneumonia (2% vs 3%). Treatment-related serious adverse events were uncommon (1% with aumolertinib; 3% with placebo), and no treatment-related deaths occurred.
Creatine phosphokinase elevation was frequent overall (reported in 60% with aumolertinib vs 14% with placebo), mostly grade 1–2 and typically asymptomatic; grade 4 events occurred in 2%, resolving with interruption and management. No interstitial pneumonia was reported.
Key Findings
- Adjuvant aumolertinib significantly improved disease-free survival (DFS) compared with placebo after complete resection in stage II–IIIB EGFR-mutated NSCLC.
- The hazard ratio for DFS was 0.17 (95% CI 0.09–0.29; p<0.0001), corresponding to an 83% relative reduction in the risk of recurrence or death.
- Median DFS was not reached in the aumolertinib group, compared with 19.4 months in the placebo group.
- The 2-year DFS rate was 88.2% with aumolertinib versus 40.6% with placebo.
- Disease recurrence occurred in 14% of patients receiving aumolertinib compared with 60% in the placebo arm.
- Distant metastasis was less frequent with aumolertinib (9% vs 39%).
- Brain metastases were observed in 6% of patients treated with aumolertinib versus 16% with placebo, although event numbers were limited.
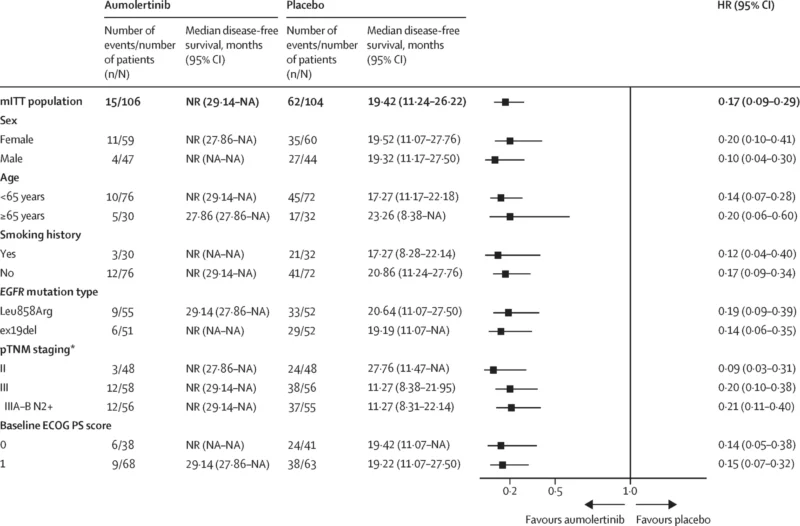
- DFS benefit was consistent across predefined subgroups, including stage, EGFR mutation subtype (ex19del vs L858R), age, sex, smoking status, and ECOG performance status.
- Overall survival data were immature at the time of analysis, with low event rates in both arms.
Conclusion
In ARTS, adjuvant aumolertinib provided substantial DFS improvement compared with placebo in resected stage II–IIIB EGFR-mutated NSCLC, with a tolerable toxicity profile compatible with long-term postoperative use. These results support aumolertinib as a strong adjuvant EGFR-targeted option in appropriately selected patients, while ongoing follow-up will clarify overall survival and long-term recurrence patterns.


