Yuji Uehara, Medical Oncologist at National Cancer Center Japan, shared a post by Timothée Olivier, Medical Oncologist at the Oncology Division of Geneva University Hospital in Switzerland, adding:
“Thrilled the first chrono-immunotherapy RCT from ASCO25 validates our JTO & JTO CRR paper: ICI infusions after 3 PM worsened outcomes in Stage III NSCLC. Fantastic to see a daring idea move so quickly to a positive RCT!”
Quoting Timothée Olivier‘s post:

“Is there a difference in outcomes between patients receiving morning versus evening chemo-immunotherapy infusion?
At ASCO25 we had the first randomized trial presented, and it was positive!
Several retrospective studies have shown that morning immunotherapy infusions are associated with longer survival. However, there was always the question of systematic bias selecting for healthier patients in morning infusions, when frailer patients may not be able to come to receive morning infusions. This could potentially explain retrospective results that may seem too good to be true…
We have recently published in The Journal for ImmunoTherapy of Cancer (available here) the biological rationale, the several retrospective studies, and the outline of what would be a pragmatic trial in that field.

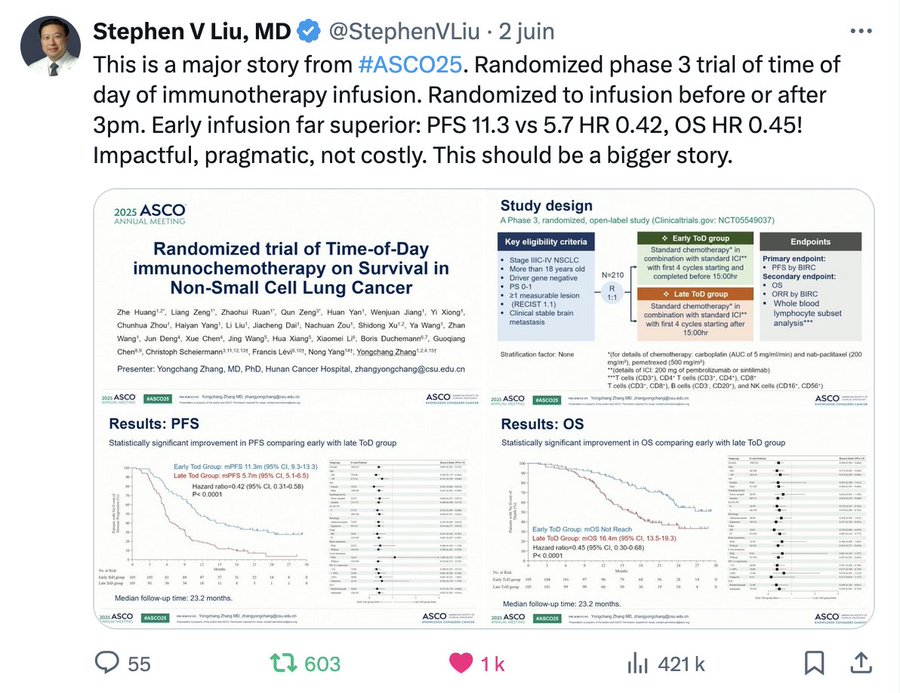
The first RCT from Pr Zhang and colleagues!
Now we have a randomized clinical trial coming from Pr Zhang and colleagues showing a hazard ratio for Overall Survival of … 0.45 ! The setting was stage IIIC-IV Non-Small Cell Lung Cancer, without driver mutation, chemo-immunotherapy first-line, and patients were randomized between therapy before 3 p.m., or after 3 pm. The primary endpoint was PFS by Blinded Assessment (BICR), OS was a secondary endpoint, as well as other biomarker endpoints.
The study design and findings are nicely summarized in a single Tweet by Stephen Liu below.
Appraisal of the data
The trial design is very clean. Of course, I would like to see the paper published to see the protocol in detail, as this is only my first take on this work. What I like a lot is that the only intervention changed is the timing of treatment, which logically gives less strength to other potential limitations as they will naturally be balanced between arms.
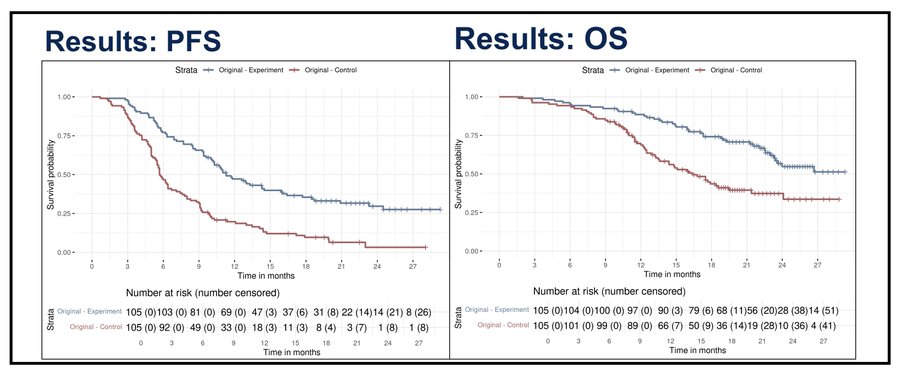
No early imbalance in censoring
The greatest challenge in this type of study is to minimize any imbalance in drop-out due to the assigned group. If patients randomly assigned to one arm disproportionately quit the trial, this could be related to how they feel, their risk of presenting the outcome, and informative censoring can then influence the observed data. For example, if patients assigned to morning infusions had quit the trial in excess, this could have retained healthier patients in that group, partly explaining the observed results. (comparable concerns to what I raised for the CHALLENGE trial here, and here).
I have digitized the curves to explore whether early censoring could have been imbalanced. As you can see below (and you can explore this yourself going to: https://www.timotheeolivier-research.com/breaking-ice and selecting “Zhang-ASCO25_PFS” or “Zhang-ASCO25-OS”), there is no patients censored in both arms, for both endpoints, during the first 9 months.
This is very reassuring because, to me, this is the most complex aspect of running such trials: ensuring that early imbalance drop-out doesn’t « undo » randomization. Unanswered questions, and the need for replication The remaining questions are:
why OS was not the primary endpoint? I would like to have more details on the statistical design, looking forward to see the publication!
will such findings replicate in non-Chinese populations?
what about immunotherapy alone? chemotherapy alone?
how the cutoff was choosen, what is the optimal time cutoff?
what about other tumor types?
The need for replication is evident in any research, and ongoing RCTs will explore this. Immunotime, led in France by Boris Duchemann, has a comparable design with Overall Survival as the primary endpoint, which is a strength (here). The Time trial, led by Zachary Buchwald will focus on ipilimumab-nivolumab in melanoma, with PFS as the primary endpoint (design here).
In Switzerland, Alfredo Addeo, Charna Dibner and Wolfram Karenovics won a TANDEM grant from ISREC on this theme in lung cancer . And maybe more on that from, let’s see!Congratulations again to Pr Zhang and his team for this impressive trial. Below is a picture with Pr Zhang, Pr Addeo, Pr Levi and myself during ASCO25 ! Looking forward to see how this story continues… Thanks for following.”
Title: Brief Report: Clinical Outcomes by Infusion Timing of Immune Checkpoint Inhibitors in Patients With Locally Advanced NSCLC
Authors: Tsuyoshi Hirata, Yuji Uehara, Taiki Hakozaki, Takayuki Kobayashi, Yuto Terashima, Kageaki Watanabe, Makiko Yomota, Yukio Hosomi
Read the Full Article.

More posts featuring Yuji Uehara.