VIKTORIA-1 is a clinical trial focused on individuals with hormone receptor-positive (HR+), HER2-negative (HER2-) Stage 3 or 4 breast cancer who have already undergone treatment with standard CDK4/6 inhibitors such as Ibrance, Kisqali, or Verzenio. This study is designed to assess the effectiveness of a new investigational therapy called gedatolisib, a dual PI3K/mTOR inhibitor, by comparing its performance against currently available treatment options tailored to patients’ tumor biology.
Participants in the trial are divided into three groups. One group receives gedatolisib in combination with Ibrance and fulvestrant, another receives gedatolisib with fulvestrant alone, and a third group receives standard-of-care treatments based on the molecular characteristics of their cancer. The aim is to determine whether gedatolisib can improve outcomes and offer a new, targeted treatment pathway for patients whose disease has progressed despite earlier therapies.
Several Oncology Professionals Have Featured the VIKTORIA-1 Trial on Social Media:
Paolo Tarantino, 2025 Yvonne’s “Top Voice” Award Winner, Research Fellow at Dana-Farber Cancer Institute:
Some thoughts: the VIKTORIA-1 results are impressive.
Yet, they come at the expense of a 3-drug regimen, with each having a distinct route of administration (IV, IM, PO) and tox. Correlative data will be key. I can’t see a future in which we treat all 2L patients with triplets.”
Antonio Giordano, Breast Medical Oncologist at Dana-Farber Cancer Institute:
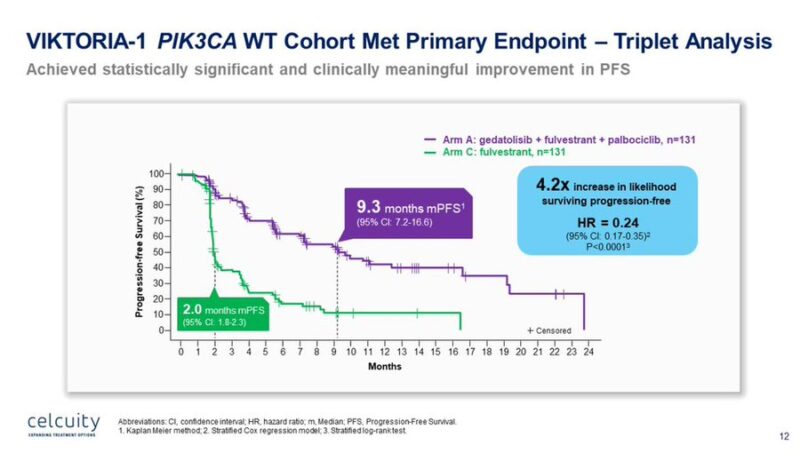
“Positive results announced from the PIK3CAwild-type cohort of Phase 3 VIKTORIA-1 trial evaluating gedatolisib + fulvestrant +/- palbociclib vs fulvestrant in HR+HER2- MBC, following progression on, or after, treatment with a CDK4/6 inhibitor and an aromatase inhibitor.”
Paolo Tarantino, 2025 Yvonne’s “Top Voice” Award Winner, Research Fellow at Dana-Farber Cancer Institute:
“Results from VIKTORIA-1 just announced by Celcuity.
The addition of gedatolisib to 2L fulvestrant/palbo improved PFS from 2 to 9.3 months (HR 0.24). Also the fulvestrant/gedatolisib combo improved PFS vs fulv mono (7.4 vs 2 mo, HR 0.33). Quite promising. ”
Amol Akhade, Consultant Medical Oncologist at Suyog Cancer Clinics:
“Did the control arm underperform in VIKTORIA-1? And how much does that matter?
The VIKTORIA-1 trial evaluated gedatolisib, a dual PI3K/mTOR inhibitor, in combination with fulvestrant ± palbociclib in HR+/HER2–, PIK3CA wild-type advanced breast cancer after CDK4/6 inhibitor progression.
The results look impressive on the surface:
Triplet arm (gedatolisib + fulvestrant + palbociclib): mPFS 9.3 months. Doublet arm (gedatolisib + fulvestrant): mPFS 7.4 months. Control arm (fulvestrant alone): mPFS 2.0 months. Delta: +7.3 months, HR = 0.24, p < 0.0001
But is the delta as clinically meaningful as it appears?
Let’s examine this through the lens of other post-CDK4/6i trials, where fulvestrant was the comparator:
Fulvestrant mPFS in Post-CDK4/6 Trials:
PACE: 4.8 months (fulvestrant alone post-CDK4/6i) DAWNA-1: 7.2 months (fulvestrant vs fulvestrant + dalpiciclib) EMERALD: 1.9–3.8 months (SOC: fulvestrant or AI, depending on ESR1 status and duration of prior CDK4/6i)
Interpretation: Most control arms deliver 3–5 months of mPFS in this setting – sometimes higher. The 2.0-month mPFS in VIKTORIA-1’s control arm is the lowest reported among these trials, raising questions about:
Selection bias toward fast progressors? Stratification imbalance? Aggressive disease biology in control arm?
Why this matters: When the control arm performs unusually poorly, the magnitude of benefit (delta) can appear inflated, even when the experimental agent is active. This is especially relevant when there’s no active comparator (e.g., elacestrant, everolimus, capecitabine) in the control arm.
That said, the HR of 0.24 is impressive – it’s hard to dismiss the signal of efficacy here. But we must balance enthusiasm with context.
What we need next:
Overall survival (OS) data, Subgroup analyses, Real-world comparator insights, Safety profile with longer follow-up.
Until then, VIKTORIA-1 adds to the growing list of post-CDK4/6 strategies, but its magnitude of benefit should be interpreted with caution – not just statistical significance, but clinical relevance.
What are your thoughts? Is this a potential game-changer or a case of an exaggerated control comparison?”
More Posts Featuring Amol Akhade, Paolo Tarantino and Antonio Giordano.


