Toni Choueiri, Director of the Lank Center for GU Oncology at Dana-Farber Cancer Institute, shared on X:
“IT IS THIS TIME OF THE YEAR AGAIN!
TOP 10 GU Bladder/Kidney Cancer TRIALS FOR 2024
1. Enfortumab Vedotin (EV) and Pembrolizumab new SOC for patients with metastatic UC. Trial EV302 displaced 30y Platinum-based combos.
PFS benefit: 12.5 vs 6.3 mo (HR= 0.45).
OS benefit: 31.5 vs 16.1 mo (HR= 0.47).

Tom Powles, ESMO2023 Plenary.
2. NIAGARA trial: Perioperative durvalumab with NAC in operable bladder cancer à EFS and OS benefit.
- 32% reduction in risk of recurrence/death.
- 25% reduction in the risk of death.
- No new safety signals.
- Potential new SOC for cisplatin-eligible MIBC!
ESMO2024 Plenary, Tom Powles again.

3. Keynote-564: 1st phase 3 trial to demonstrate overall survival benefit for adjuvant treatment in RCC Pembrolizumab.
- 38% reduced risk of recurrence/death (HR 0.62).
- New SOC for high-risk RCC post-nephrectomy.
NEJM with ASCO GU 2024.

4. LITESPARK-005: Phase 3 trial that led to Belzutifan, US FDA approval in advanced pretreated ccRCC.
- PFS benefit: 18-mo PFS, 24% vs 8.3% (P=0.002).
- ORR benefit: 21.9% vs 3.5% (P<0.001).
5. And now Neeraj Agarwal with a practice-informing trial for Belzutifan in RCC.
More is not always better! The efficacy of belzutifan was similar between the 120 mg dose and the 200 mg dose for previously treated ccRCC.
6. TiNivo-2: The addition of nivolumab (PD1) to Tivozanib did not result in improved clinical outcomes in patients with mRCC treated with prior PD1.
Results expand on conclusion from CONTACT03.
Presented ESMO2024 and published in The Lancet.

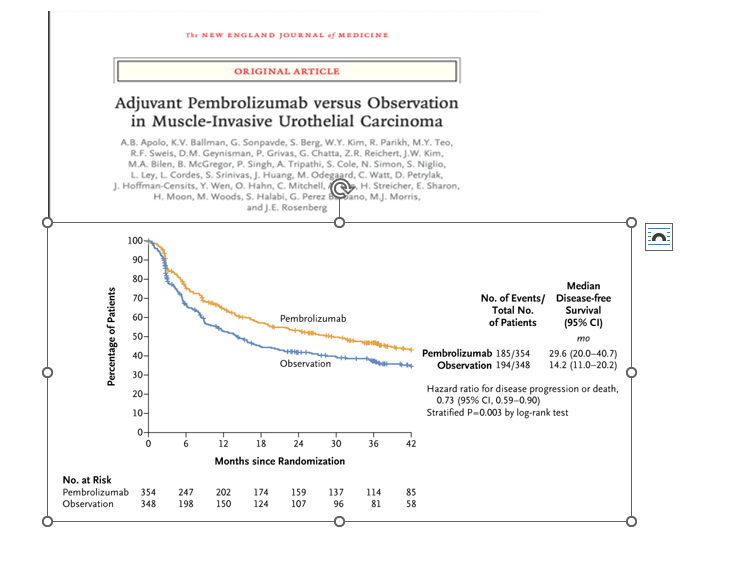
7. AMBASSADOR Trial: In patients with MIBC, adjuvant pembrolizumab resulted in DFS benefit (Median DFS 29.6 vs 14.2 months) over observation.
Andrea Apolo, NEJM.
8. Promising data by Hedyeh Ebrahimi, Nazli Dizman, Luis Mezasco, and super mentor Sumanta K. Pal, looking at microbiome modulation in RCC with CBM588 (Live Bacterial Product) and showing the potential to enhance clinical outcomes in treatment-naive mRCC patients on cabozantinib and nivolumab.
This study follows on an earlier one also in Nature Medicine with Nivo/Ipi.

9. Promising data from ZIRCON trial à Zr-girentuximab PET CT imaging for detection and characterization of ccRCC with High accuracy with a favorable safety profile.

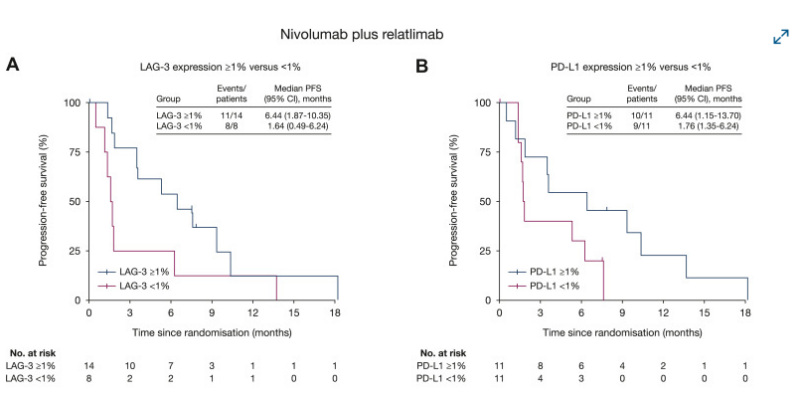
10. 10/FRACTION-RCC: Phase 2 trial evaluating dual checkpoint inhibition with NIVO + RELA or IPI in IO-naïve advanced RCC patients.
Antitumor activity and manageable safety profile with NIVO +RELA.
Interesting results for LAG-3/PD-L1 IHC in NIVO +RELA.
Toni Choueiri is the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute (DFCI), co-leader of the Kidney Cancer Program at Dana-Farber/Harvard Cancer Center, and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School.
As a medical oncologist, clinical trialist, and translational researcher, he specializes in treating genitourinary cancers (prostate, bladder, testis, and kidney cancer), with a focus on kidney cancer.


