Erman Akkus, Medical Oncology Fellow at Ankara University, shared on X:
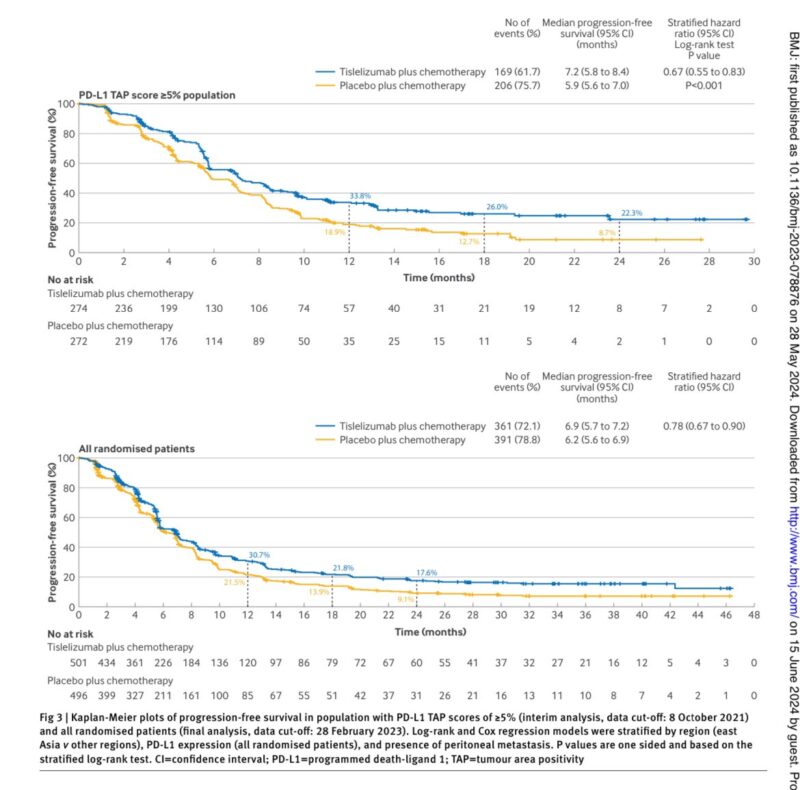
“Tislelizumab plus chemotherapy vs chemotherapy alone in first-line treatment of advanced gastric/gastroesophageal junction adenocarcinoma.
RATIONALE-305
- Median overall survival (mOS) in the intention-to-treat (ITT) population: 15 months versus 12.9 months, with a hazard ratio (HR) of 0.80 (95% CI 0.70 to 0.92); P=0.001.
- Median overall survival (mOS) in the population with PD-L1 Tumor Proportion Score (TPS) ≥5%: 16.4 months versus 12.8 months, HR: 0.71 (95% CI 0.58 to 0.86).”
Authors: Miao-Zhen Qiu, Do-Youn Oh, Ken Kato, Tobias Arkenau, Josep Tabernero, Marcia Cruz Correa, Anastasia V Zimina, Yuxian Bai, Jianhua Shi, Keun-Wook Lee, Jufeng Wang, Elena Poddubskaya, Hongming Pan, Sun Young Rha, Ruixing Zhang, Hidekazu Hirano, David Spigel, Kensei Yamaguchi, Yee Chao, Lucjan Wyrwicz, Umut Disel, Roberto Pazo Cid, Lorenzo Fornaro, Ludovic Evesque, Hongwei Wang, Yaling Xu, Jiang Li, Tao Sheng, Silu Yang, Liyun Li, Markus Moehler, and Rui-Hua Xu.

 Source: Erman Akkus/X
Source: Erman Akkus/X


