Timothée Olivier, Medical Oncologist at HUG – Geneva University Hospitals, shared a post by the New England Journal of Medicine on X, adding:
“NATALEE trial, adjuvant ribociclib 21 days/28 for… 3 years!
Cost alert! Financial toxicity.
Assuming the iDFS gain is reliable (which I questioned here) .. the estimated cost to avert ONE iDFS event would be $ 11,200,000!
Here is the calculation.
Firstly, let’s estimate the NNT (number needed to treat), which is the number of patients to be treated to avert one iDFS event.
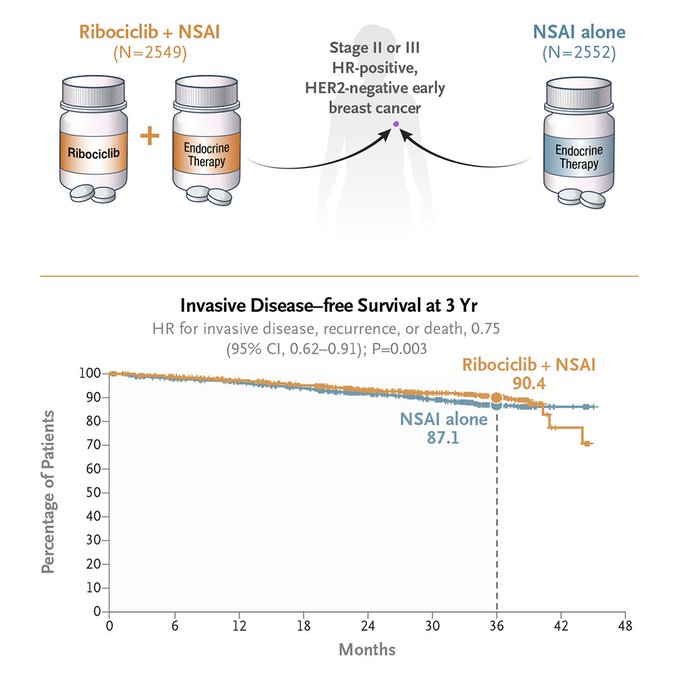
In the NATALEE trial, the 3-y absolute iDFS gain is 3.3 point-percentage (87.1% to 90.4%).
NNT = 100/3.3 = 30.3.
Secondly, let’s estimate the cost for one patient treatment:
– median exposure to ribociclib alone = 27 months
– 27 months = 821 days = 29,3 cycles of 21 days/28 of ribociclib
– minimum unit Average Whole Price for 200 mg of ribociclib according to RedBook = 336,86 USD.
21.9% of patients experienced ≥ 1 dose reduction (max reduction = 200 mg/day).
I assumed (very conservative estimate)
– 21.9% pts took the 200 mg dose during 27 months.
– the remaining 78.1% took the 400 mg dose (27 months).
—> average estimated cost per patient = 369 422,30 USD.
Now: multiply the NNT by the estimated average cost per patient $11 200 000 USD.
At VK Prasad Laboratory we published a similar analysis of recent approvals and the maximum was $2 640 000.
With the NATALEE strategy, it is more than 4 times this number!

Financial toxicity is a real burden for patients and for society… Will Novartis lower the price if this indication is approved? Many published works on this topic, and more to come. VK Prasad Laboratory and Vinay Prasad thanks for following.”
Quoting the New England Journal of Medicine’s post:
“In patients with stage II or III early breast cancer, the addition of ribociclib to adjuvant hormonal therapy resulted in a significant improvement in 3-year invasive disease–free survival.
Read the full NATALEE trial results and Research Summary here.”
Source: Timothée Olivier/X and New England Journal of Medicine/X