Talha Badar, Hematology/Oncology specialist in Mayo Clinic, shared a post on X:
“For next few weeks, we will review controversies in the management of acute leukemia and myeloid disorders.
Starting with ‘Post-transplant FLT3 maintenance therapy for AML’
Key points:
How should we define effectiveness of maintenance therapy post allo-HCT.
Who are the patients that can benefit the most from maintenance therapy.
What should be the duration of maintenance therapy post allo-HCT.
Factors that influence decision for maintenance therapy:
- High risk disease at diagnosis.
- MRD positivity prior to allo-HCT.
- Conditioning intensity (RIC vs MAC: ↑ relapse).
- Therapeutic agent: targeted agent against mutation, toxicity profile.
- Post allo-HCT complication; limiting maintenance therapy option.
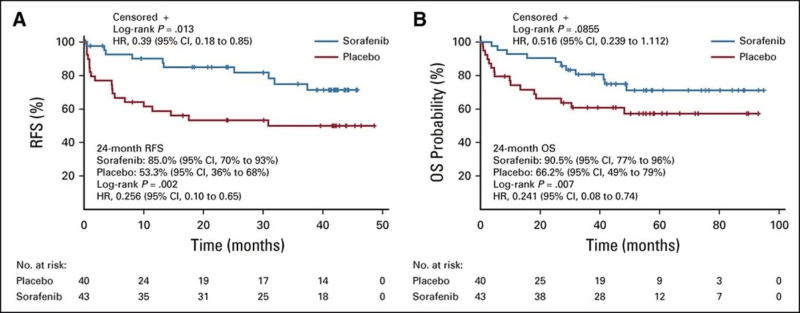
SORMAIN: Randomized PII study on 83 FLT3-ITDm AML patients in CHR after allo-HCT, assigned sorafenib (n=43) or placebo (n=40) for 24 mo.
- PE: RFS
- Median f/u of 41.8 mo, HR 0.39 (95% CI 0.18-0.85, p 0.013) favored sorafenib in improving RFS.
All patients received IC, FLT3i (excluding sorafenib was allowed)9/83 received midostaurin during induction
- No CHR or MRD+ pre-HCT, ×improve RFS
- MRD- pre-HCT, improved RFS
- MRD+ post HCT, improved RFS
- MRD- post HCT × improve RFS
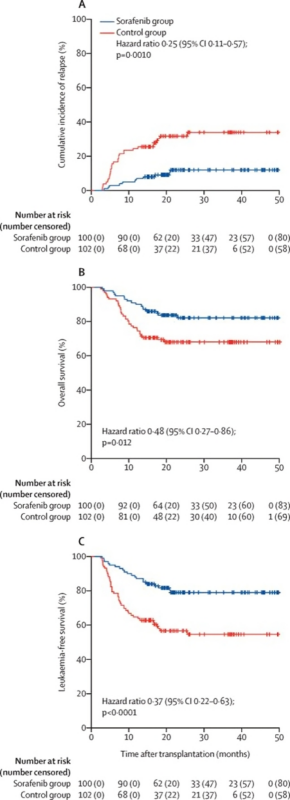
FLT3i maintenance, PIII study (Xuan et al. Lanc Onc 2020)
- Pts 18-60 yrs, in cCR pre and post allo-HCT and had count recovery at D60.
- Randomized to receive sorafenib (n=100) for 6 mo or control (n=102).
- PE: 1-yr cumulative incidence of relapse was met: HR 0.25 (95% CI, 0.11-0.57, p= 0.0010).
RADIUS trial (Midostaurin)
- Randomized PII study on 60 FLT3-ITDm AML pts in CR1 pre allo-HCT, assigned midostaurin (n=30) or control (n=30) for 12 mo.
- PE: RFS was not met
FLT3i maintenance post allo-HCT in AML
MORPHO trial (Gilteritinib) PIII study on 356 FLT3-ITDm AML pts in CR1 pre allo-HCT, free of GVHD assigned gilteritinib (n=178) or control (n=178) for 24 mo.
- PE; RFS, SE; OS and impact of MRD pre- and post allo-HCT on RFS/OS.
MORPHO trial (Gilteritinib) PIII study on 356 FLT3-ITDm AML pts in CR1 pre allo-HCT, free of GVHD assigned gilteritinib (n=178) or control (n=178) for 24 mo.
- PE; RFS, SE; OS and impact of MRD pre- and post allo-HCT on RFS/OS.
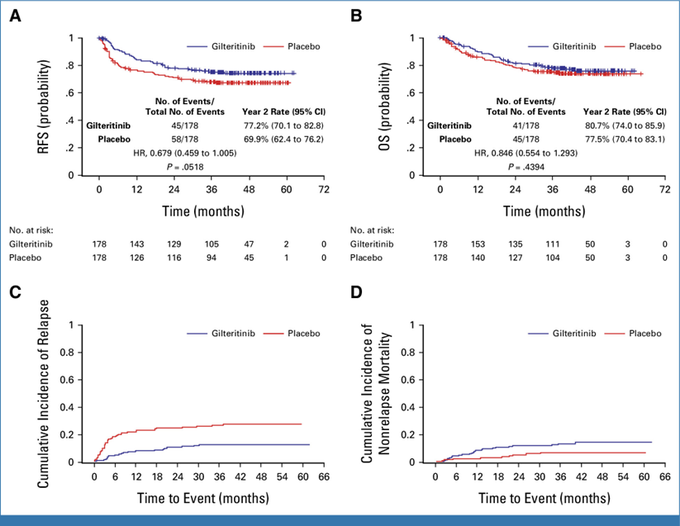
MORPHO trial (Gilteritinib)
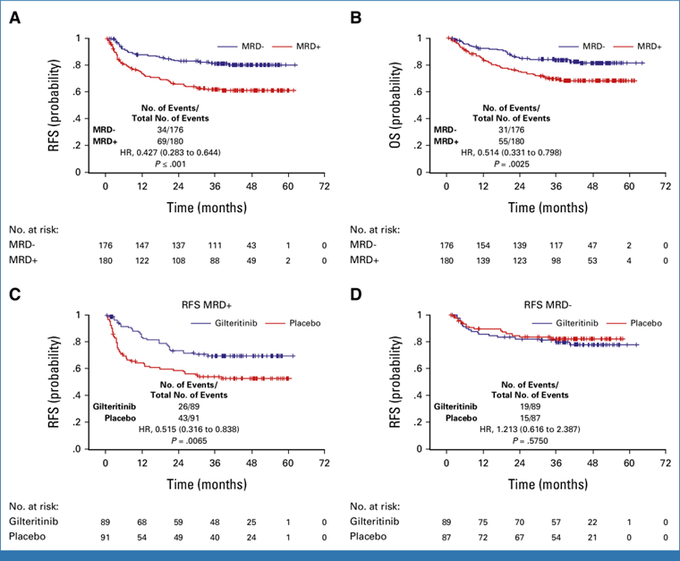
Secondary analysis
Impact of molecular MRD (PCR-NGS) at a level of ≥ 1 x10-6 pre- or post-HCT.
Gilteritinib showed benefit for RFS in MRD+.
Significant AEs were mainly myelosuppression.
Fig A/B; MRD peri-HCT irrespective of Rx arm, Fig C/D MRD peri-HCT based on Rx.
Conclusion
- Data is heterogeneous; (a) assessment of MRD, (b) use of FLT3i during induction (c) concurrent mutation analysis/data was not available on most studies which could impact benefit with FLT3i.
- Duration of FLT3i post allo-HCT: Sorafenib showed benefit; whether 6 mo or 24 mo post allo-HCT?
- More potent FLT3i only showed benefit on subset of pts with MRD, did the benefit with Sorafenib driven by multi-kinase activity?”
More posts featuring Talha Badar.
Dr. Talha Badar, MD, is a specialist in Hematology Oncology based in Mayo Clinic, Jacksonville, Florida. His primary areas of expertise include Leukemia, particularly Acute Myeloid Leukemia (AML), Myelodysplastic Syndrome (MDS), Acute Lymphoblastic Leukemia (ALL), and Bone Marrow Transplantation. Over his career, he has actively contributed to clinical research and clinical trials.


