Patrick Forde, Professor of Thoracic Oncology at Johns Hopkins University, shared a post on X by Joshua Reuss, Thoracic Medical Oncologist at Georgetown Lombardi Comprehensive Cancer Center, adding:
“Great thread, proud of Joshua Reuss and many pt/colleagues in bringing to fruition this neoadjuvant immunotherapy trial for mesothelioma with cutting edge liquid biopsy data from Elsa Anagnostou, now open access Nature Medicine.”
Quoting Joshua Reuss‘s post:
“Honored to present our data on neoadjuvant nivolumab and nivolumab/ipilimumab in potentially resectable diffuse pleural mesothelioma (DPM) at WCLC25.
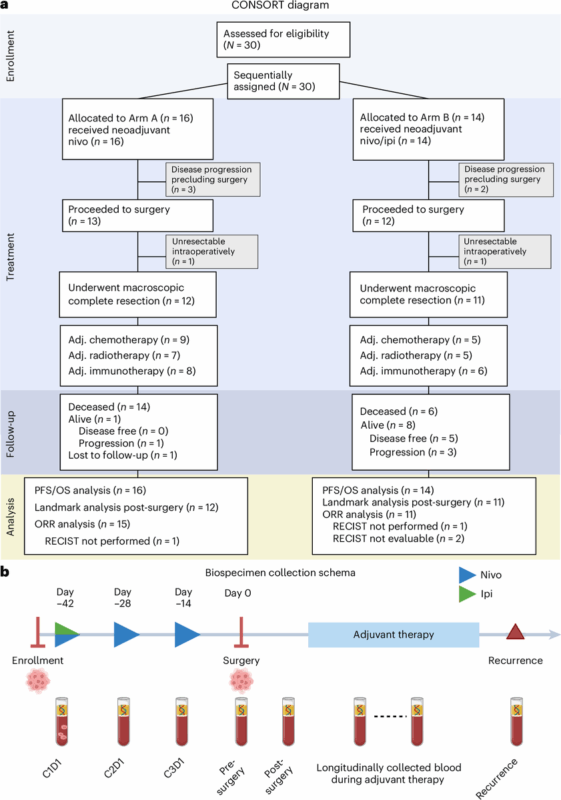
We evaluated perioperative nivo and nivo/ipi in epithelioid or biphasic DPM deemed resectable by multi-D assessment.
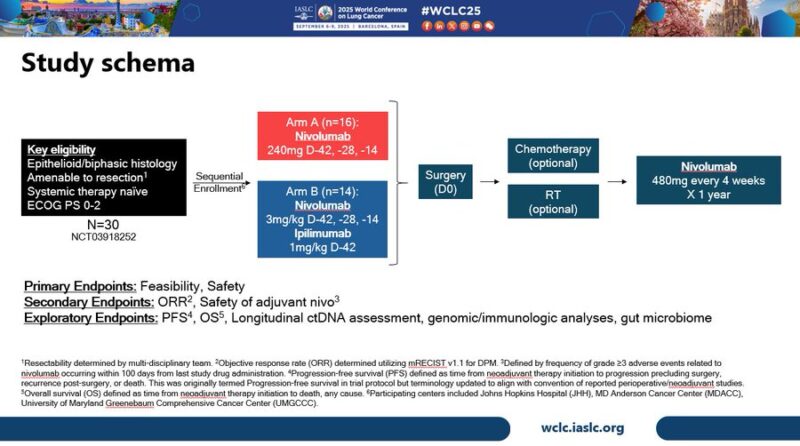
Neoadjuvant Rx was safe & feasible, with 81.3% of patients in the nivo arm proceeding to surgery and 75% undergoing resection. In nivo/ipi arm, 85.7% of patients proceeded to surgery, and 78.6% underwent resection. one DLT was observed in each arm, neither prevented surgery. 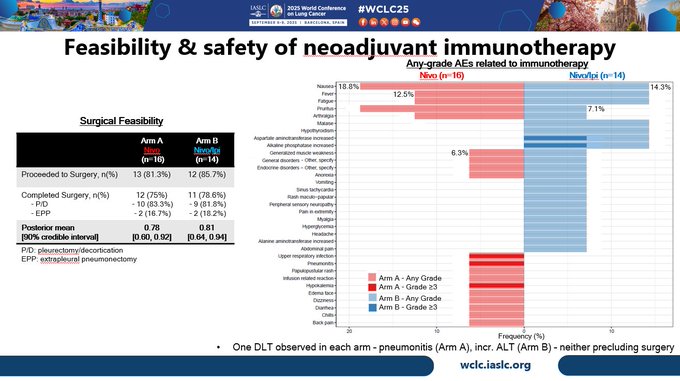
Efficacy was exploratory, but at med f/u of 43.2mo in nivo arm, mPFS and mOS were 9.6mo and 19.3mo. in nivo/ipi arm, at med f/u of 24.1mo, mPFS and mOS were 19.8mo and 28.6mo. in this arm 8/14 pts were alive and 5 were disease-free at data cutoff. 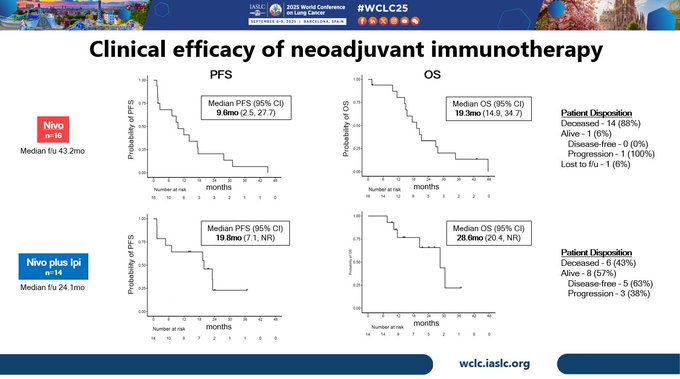
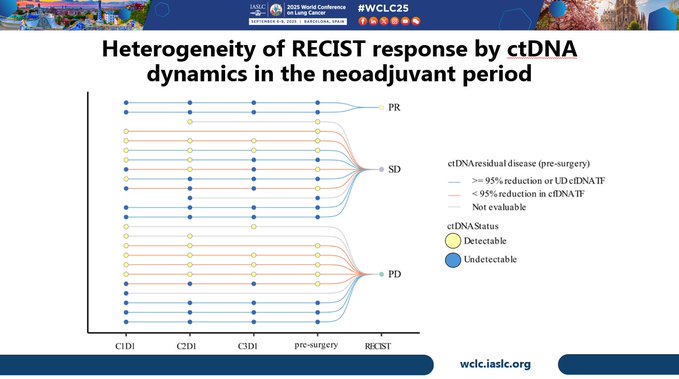
Exploratory ultra-sensitive ctDNA analyses led by Elsa Anagnostou showed that all patients who were not able to undergo surgery had ctDNA detected prior to planned resection, with increased rate of detection pre-Rx and pre-surgery compared to those who completed surgery.
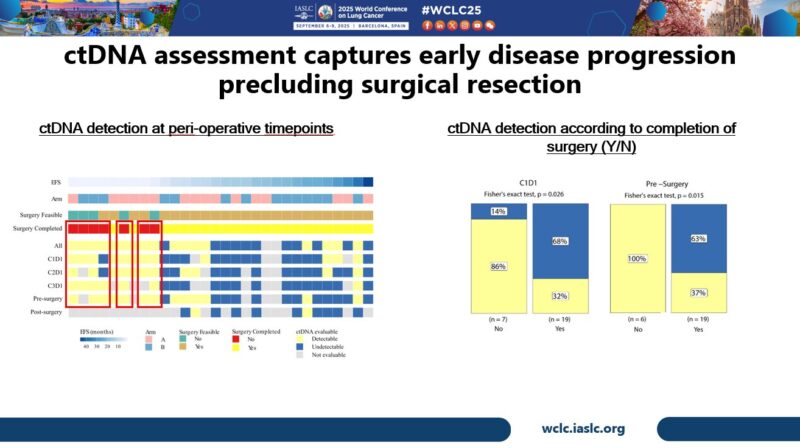
ctDNA dynamics were markedly heterogeneous when classified by imaging response category, highlighting challenges of RECIST response assessment in DPM. Also, both undetectable ctDNA prior to surgery, & >95% reduction of ctDNA prior to surgery was associated with favorable PFS.
For more information, please see our simultaneous open access publication in Nature Medicine, where you can learn more about other clinical endpoints and ctDNA analyses from our trial.
I am indebted to my fellowship mentors Drs. Julie Brahmer and Patrick Forde, as well as all members of Johns Hopkins Thoracic Oncology. thanks also to MD Anderson Cancer Center and UMGCCC, and especially the patients who entrusted their care to our team.”
More from Patrick Forde on OncoDaily.


