Long-Term Benefits of Dabrafenib-Trametinib in Stage III Melanoma: 8-Year COMBI-AD Trial Results .
Authors: Georgina V. Long, Axel Hauschild, Mario Santinami, John M. Kirkwood, Victoria Atkinson, Mario Mandala, Barbara Merelli, Vanna Chiarion Sileni, Marta Nyakas, Andrew Haydon, Caroline Dutriaux, Caroline Robert, Laurent Mortier, Jacob Schachter, Dirk Schadendorf, Thierry Lesimple, Ruth Plummer, James Larkin, Monique Tan, Sachin Bajirao Adnaik, Paul Burgess, Tarveen Jandoo and Reinhard Dummer.
Published in The New England Journal of Medicine on June 19, 2024
Introduction:
The COMBI-AD trial investigated the long-term efficacy and safety of adjuvant dabrafenib plus trametinib in patients with resected stage III melanoma harboring BRAF V600 mutations. This combination therapy has become a standard treatment option, but extended follow-up data were needed to assess its impact on overall survival and other key outcomes.
Design/Methods:
This randomized, double-blind, placebo-controlled phase 3 trial enrolled 870 patients with completely resected stage III melanoma with BRAF V600 mutations. Participants were randomly assigned to receive either 12 months of dabrafenib (150 mg twice daily) plus trametinib (2 mg once daily) or matched placebos.
The primary endpoint was relapse-free survival, with overall survival, distant metastasis-free survival, and safety as key secondary endpoints. The final analysis was conducted after a median follow-up of 8.33 years for the combination therapy group and 6.87 years for the placebo group.
What We Learned:
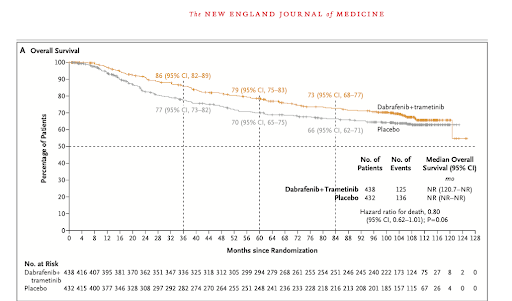
- While not statistically significant, dabrafenib plus trametinib showed a trend towards improved overall survival compared to placebo (hazard ratio for death: 0.80; 95% CI: 0.62-1.01; p=0.06).
- The combination therapy demonstrated a more pronounced survival benefit in patients with BRAF V600E mutations (91% of participants) (hazard ratio for death: 0.75; 95% CI: 0.58-0.96).
- Dabrafenib plus trametinib significantly improved relapse-free survival (hazard ratio: 0.52; 95% CI: 0.43-0.63). The median relapse-free survival was 93.1 months in the combination group versus 16.6 months with placebo.
- The combination therapy also improved distant metastasis-free survival (hazard ratio: 0.56; 95% CI: 0.44-0.71).
- At 10 years, estimated relapse-free survival was 48% with dabrafenib plus trametinib versus 32% with placebo. Distant metastasis-free survival at 10 years was 63% versus 48%, respectively.
- No new long-term safety concerns were identified during the extended follow-up period.
Key Highlights:
- Subgroup analyses suggested greater benefits in patients with BRAF V600E mutations, primary tumor ulceration, and macrometastasis.
- The combination therapy showed consistent benefits across most subgroups, except for patients with BRAF V600K mutations, where the trend for overall survival was reversed.
- Subsequent anticancer therapies were used more frequently in the placebo group (57%) compared to the combination therapy group (42%).
- In the combination therapy group, patients who received subsequent immunotherapy had longer median overall survival than those who received subsequent BRAF-targeted therapy (104.6 months vs. 41.8 months).
- The incidence of new primary or secondary cancers was slightly higher in the combination therapy group, primarily observed in the first 3 years of follow-up.
Key Takeaway Messages:
- Adjuvant dabrafenib plus trametinib provides long-term benefits in relapse-free survival and distant metastasis-free survival for patients with resected stage III BRAF V600-mutant melanoma.
- While overall survival improvement did not reach statistical significance, there was a clear trend towards benefit, particularly in patients with BRAF V600E mutations.
- The combination therapy demonstrated a manageable long-term safety profile with no new concerns identified during extended follow-up.
- Subgroup analyses suggest that certain patient characteristics, such as BRAF mutation type and disease features, may influence the magnitude of benefit from adjuvant targeted therapy.
- The impact of subsequent therapies on overall survival highlights the importance of considering the entire treatment landscape when interpreting long-term outcomes.
- These results provide valuable insights for treatment decision-making in stage III melanoma, particularly for patients with BRAF V600E mutations.
In conclusion, the COMBI-AD trial’s final analysis reinforces the role of adjuvant dabrafenib plus trametinib in improving long-term outcomes for patients with resected stage III BRAF V600-mutant melanoma.
While questions remain about its impact on overall survival and its comparative efficacy to other adjuvant options, these results provide a solid foundation for personalized treatment strategies in this patient population.
Summary by Amalya Sargsyan, MD
Final Results for Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma


