
Gustave Roussy Research Shares a Study on TALAPRO-2 as a New Standard of Care in mCRPC
Gustave Roussy Research posted on LinkedIn:
”TALAPRO-2 sets a new standard of care in metastatic castration-resistant prostate cancer (mCRPC)
Two original research articles published in The Lancet (July 16, 2025), authored by Professor Karim Fizazi and an international team of investigators, report the final overall survival results of the phase 3 TALAPRO-2 trial. The findings establish a new benchmark in the treatment of mCRPC.
Why it matters
mCRPC remains one of the deadliest forms of prostate cancer. Around 25% of patients have homologous recombination repair (HRR) gene alterations, particularly BRCA1/2, associated with poor prognosis but increased sensitivity to PARP inhibitors.
What was done
TALAPRO-2 is a phase 3, randomized, double-blind trial evaluating talazoparib (a PARP inhibitor) + enzalutamide versus enzalutamide alone as first-line therapy in two cohorts: an unselected population and a group with HRR gene alterations.
Key findings
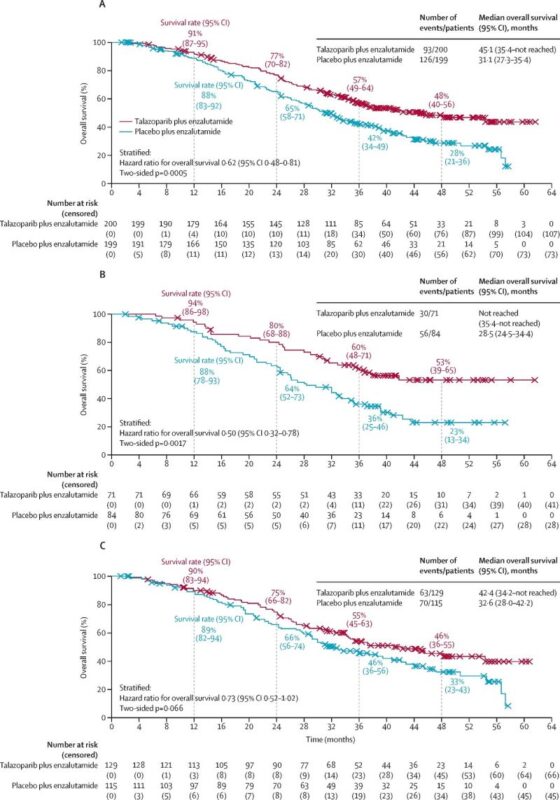
In the HRR-deficient cohort, the combination significantly improved overall survival (OS):
– 45.1 months vs 31.1 months (HR 0.62; p=0.0005)
– In BRCA1/2-mutated patients: 50% reduction in risk of death (HR 0.50; p=0.0017)
In the overall population:
– 45.8 months vs 37.0 months (HR 0.80; p=0.016)
The safety profile was manageable, and quality of life was preserved.
How it works
PARP inhibitors like talazoparib block DNA repair in cancer cells with HRR deficiencies. Combined with enzalutamide, which targets the androgen receptor pathway, this dual strategy disrupts two essential tumor survival mechanisms.
Conclusion
TALAPRO-2 is the f
irst phase 3 trial to demonstrate a significant OS benefit with a PARP inhibitor + ARPI combination, in both biomarker-selected and unselected mCRPC patients. It reinforces the importance of genomic testing and offers a promising first-line therapeutic option.
Published in The Lancet (July 16, 2025):
HRR-deficient cohort:
Title: Talazoparib plus enzalutamide in men with HRR-deficient metastatic castration-resistant prostate cancer: final overall survival results from the randomised, placebo-controlled, phase 3 TALAPRO-2 trial
Authors: Karim Fizazi, Arun A Azad, Nobuaki Matsubara, Joan Carles, Prof André P Fay, Ugo De Giorgi, Jae Young Joung, Peter C C Fong, Eric Voog, Prof Robert J Jones, Neal D Shore, Curtis Dunshee, Stefanie Zschäbitz, Jan Oldenburg, Dingwei Ye, Xun Lin, Matko Kalac, A Douglas Laird, Dana Kennedy, Prof Neeraj Agarwal
Read the Full Article here.
Overall population:
Title: Talazoparib plus enzalutamide in men with metastatic castration-resistant prostate cancer: final overall survival results from the randomised, placebo-controlled, phase 3 TALAPRO-2 trial
Authors: Neeraj Agarwal, Arun A Azad, Joan Carles, André P Fay, Nobuaki Matsubara, Cezary Szczylik, Ugo De Giorgi, Jae Young Joung, Peter C C Fong, Eric Voog, Robert J Jones, Neal D Shore, Fred Saad, Curtis Dunshee, Stefanie Zschäbitz, Jan Oldenburg, Xun Lin, Cynthia G Healy, Matko Kalac, Dana Kennedy, Karim Fizazi
Read the Full Article here.
Overall survival in patients with (A) any HRR gene alteration; (B) BRCA1/2 gene alterations; and (C) non- BRCA1/2 HRR gene alterations (HRR-deficient intention-to-treat population). HRR=homologous recombination repair (source : The Lancet).”
More posts featuring TALAPRO-2 on OncoDaily.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
