Amar Kelkar, Stem Cell Transplantation Physician at the Dana-Farber Cancer Institute, posted on X/Twitter:
“Today in Annals of Int Med: We show Axi-cel and Liso-cel are NOT COST-EFFECTIVE in our decision analysis of second-line CAR-T therapies for Diffuse Large B-Cell Lymphoma, and calculate the economic impact to be >$6.8 billion over 5 years!
2/Only 20-30% with relapsed/refractory DLBCL achieve durable remissions with standard-of-care second-line salvage chemoimmunotherapy with autologous stem cell transplantation (ASCT). Novel CAR-T therapies improve survival over ASCT but cost >$400k/infusion!
3/We used a microsimulation model to evaluate if axi-cel and liso-cel are cost-effective for DLBCL second-line treatment compared to ASCT. This allowed us to model real-world situations to predict lifetime costs and lifetime effectiveness for 10,000 simulated patients.
4/We modeled:
2nd to 5th line therapies for DLBCL using real-world practice patterns
Costs: US dollars
Effectiveness: Measured in quality-adjusted life-years
Discounting Rate: 3%/year (present costs & effectiveness more valuable than theoretical future costs & effectiveness)
5/There is no prescribed value cutoff in the US, so we modeled for a very high willingness-to-pay (WTP) threshold of $200,000/QALY to reflect decision-makers’ willingness to spend more on oncology therapies. Countries that utilize WTP use a lower number (~$50,000-100,000/QALY).
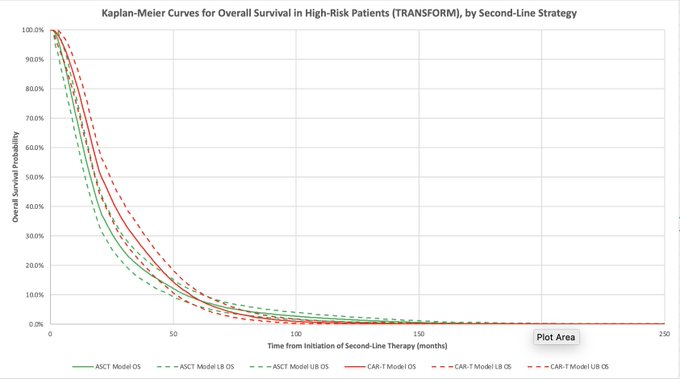
7/The model confirmed improved survival and quality of life with axi-cel and with liso-cel compared to ASCT! Median overall survival increased by 4 months for axi-cel and 1 month for liso-cel.
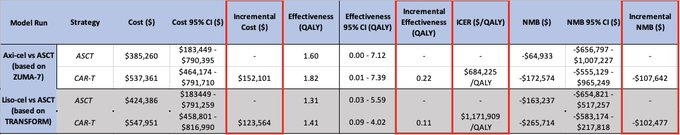
8/Incremental Cost-Effectiveness Ratios (ICER) were staggeringly high for second-line CAR-T in DLBCL: $684,225/QALY for axi-cel and $1,171,909/QALY for liso-cel (vs ASCT)—far above our pre-specified $200,000/QALY WTP threshold, making both axi-cel and liso-cel NOT COST-EFFECTIVE!
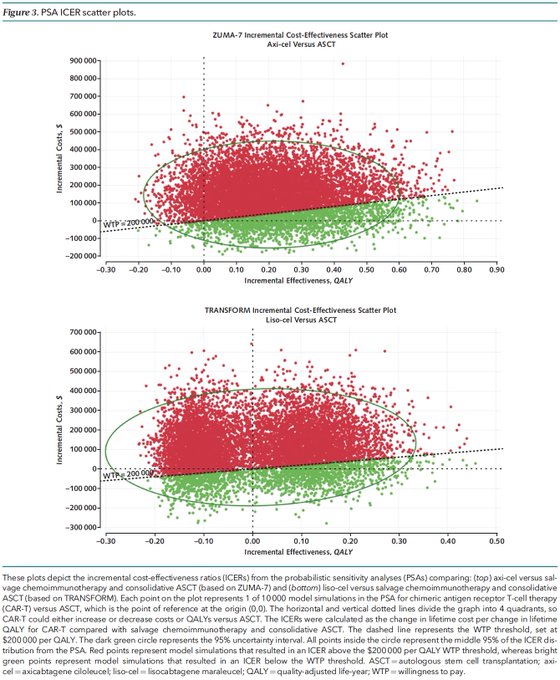
9/Probabilistic sensitivity analyses of the model (which varied individual parameters) for second-line CAR-T vs ASCT for DLBCL showed that in the majority of simulations, axi-cel and liso-cel were still NOT COST-EFFECTIVE!
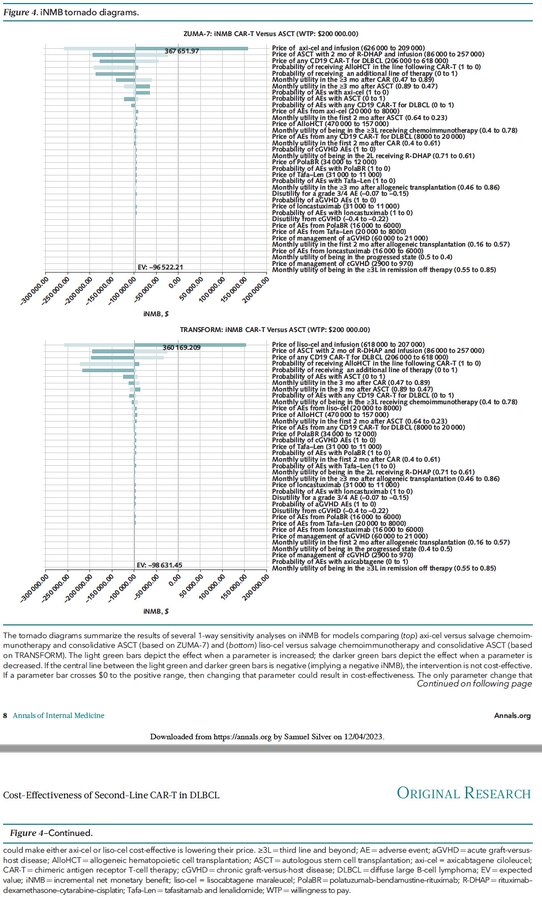
10/Sensitivity analysis showed that ONLY LOWERING PRICES of second-line CAR-T could make them cost-effective compared to ASCT for DLBCL: axi-cel and liso-cel prices must drop to $321,123 and $313,730 to be cost-effective—a LARGE reduction from current prices ($417,735 & $412,362)!
11/Budget Impact Analysis: Treatment of high-risk patients with DLBCL with 2nd-line CAR-T (over ASCT) adds $6.7-6.8 billion to US healthcare spending over 5 yrs! With >1000 active CAR-T trials under study, the impact of broad adoption of these therapies is astronomical!







