Marco Donia, Clinical Research Associate Professor at University of Copenhagen, shared on LinkedIn:
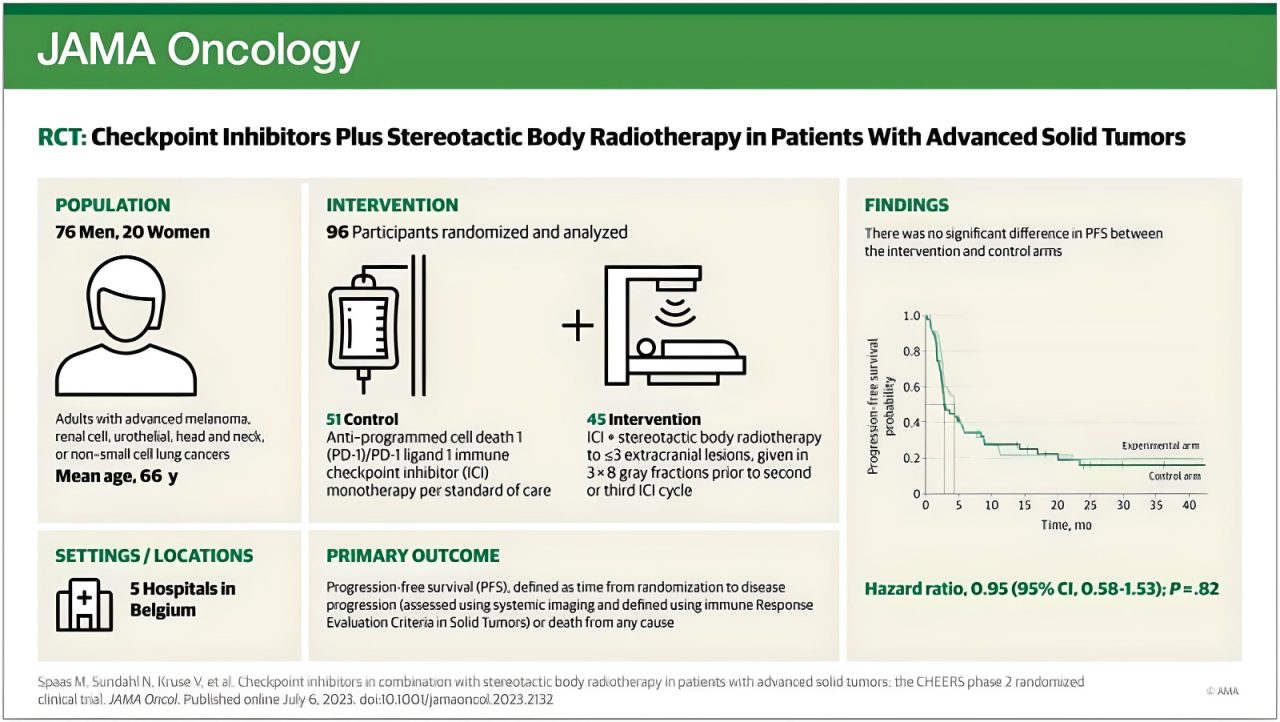
“Adding Stereotactic Body Radiotherapy to anti-PD-1/PD-L1: safe but no added clinically meaningful benefit in unselected tumors.
Published in JAMA Oncology at here.
- No meaningful improvement in PFS, OS, or ORR was observed
- No unexpected signals of added toxicity
In a multicenter (5 Belgian hospitals) phase 2 randomized clinical trial, the group of Piet Ost enrolled 99 patients with unselected solid tumors to receive anti–PD-1/PDL-1 alone as per standard of care (control arm) or combined with stereotactic body radiotherapy (SBRT) 3 × 8 gray to a maximum of 3 lesions before the second or third immunotherapy cycle.
- Potential abscopal effect, not observed in this study in a clinically meaningful fashion.
- Heterogeneous, heavily pre-treated (including ~50% having received previous Radiotherapy), patient population
- Unclear whether enrolling patients with one single tumor type (melanoma? NSCLC? other?) can make a difference.
My Take Home:
Although early promising case reports and single-cohort studies suggested the abscopal effect of radiotherapy and potential synergism between immunotherapy and radiotherapy (reviewed at Future Medicine), this randomized study conducted in patients with unselected tumor types did not confirm them. SBRT + immunotherapy in unselected tumor types did not appear a good strategy for improving immunotherapy outcomes. However, patients undergoing immunotherapy can safely receive SBRT upon indication.”
For more details click here.
Source: Marco Donia/Linkedin