Nina Niu Sanford, Chief of Gastrointestinal Radiation Oncology Service at UT Southwestern Medical Center, shared on Twitter:
“Out now in Journal of Clinical Oncology! Our work on clinical trial endpoints and inclusion criteria in GI oncology trials, and how we can better leverage RCTs to demonstrate the value of RT going forward. With William A Hall and Ted Hong.
Brief thread below.
First, we looked at all cooperative group GI trials approved/activated since 2006 (when GI steering committee formed). N=65 total (12 RT, 49 systemic). We found major differences in trial endpoints by modality: 83% of RT trials had OS as primary endpoint vs. 32% of systemic.
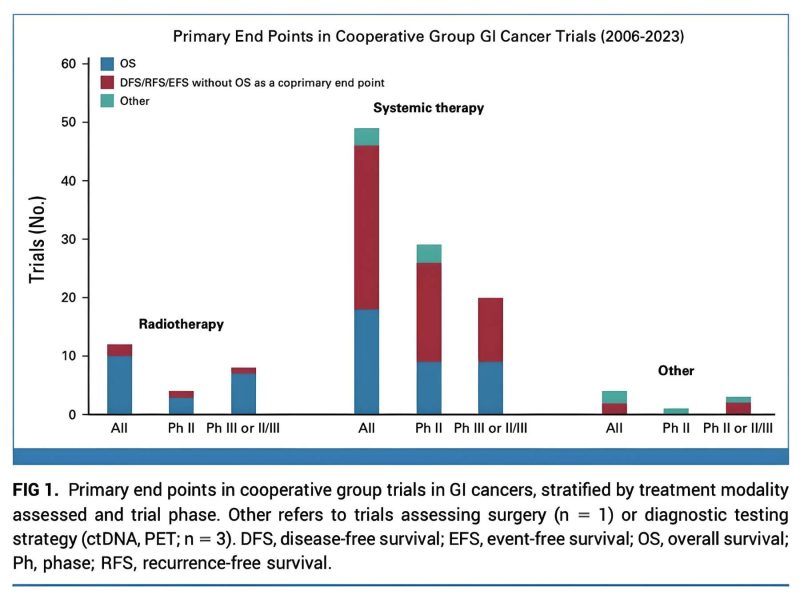
Differences persisted when stratifying by trial Phase. We also found differences in inclusion criteria, with more systemic trials being limited to smaller patient subsets vs. most RT trials were for all comers, at diagnosis.
All RT trials (except 1, RTOG 1112) have been negative. Our argument is that RT will not improve OS in unselected patients. Thus, future trials in GI cancers should consider the following:
1) Incorporate new patient-centered endpoints. Arguably, RT does not improve OS in most neoadjuvant or adjuvant settings. However, patients also value QOL – sometimes as much, or even more than OS. We believe that RT can improve QOL……but QOL has historically been poorly captured and often using long, burdensome instruments. Future trials should include more specific QOL endpoints such as time toxicity, financial toxicity, and QOL should continue to be measured at progression and until death.
2) Refine trial inclusion criteria. Most RT trials in GI cancers have broad inclusion criteria. This makes no sense for a local modality such as RT, since the predominant form of disease progression for locally advanced GI cancers is distant.
Thus, RT trials need to more selective – the way surgeons select patients for a Whipple, or med oncs choose patients for targeted therapy. Our final paragraph summarizes it all.

Huge thanks to the JCO team, the 3 great reviewers, my friend and colleague Manju George, and of course to my mentors William A Hall and Ted Hong. Really excited for this paper to come out. Please read!”
For more details click here.
Source: Nina Niu Sanford/Twitter


