Toni Choueiri shared a post on X:
“JUST IN: US_FDA approved nivolumab with platinum-doublet chemotherapy as neoadjuvant treatment, followed by single-agent nivolumab after surgery as adjuvant treatment, for resectable (tumors ≥ 4 cm/node +) NSCLC.
Efficacy based on CHECKMATE-77T: led by Dr Tina Cascone, MD Anderson Cancer Center: Median EFS was not reached in the nivo arm and 18.4 months in the chemotherapy arm (HR 0.58 [95% CI: 0.43, 0.78].”
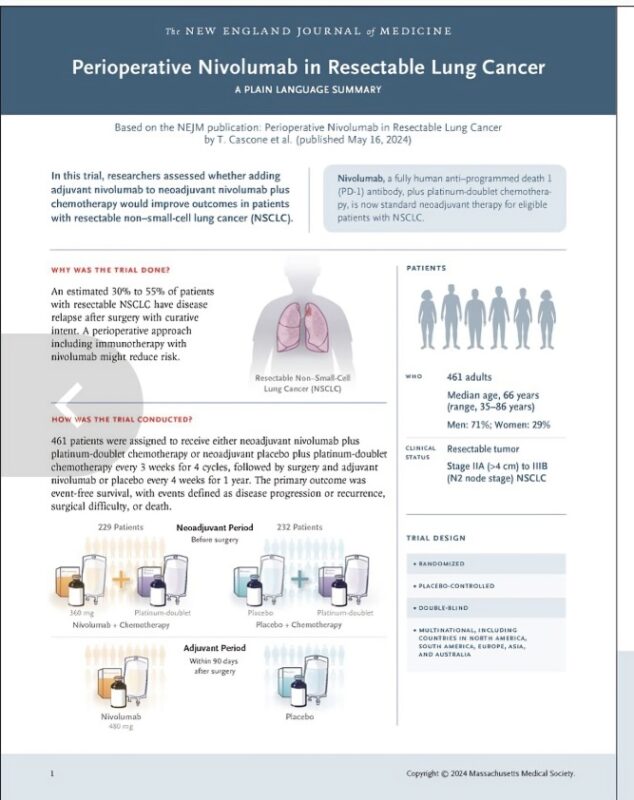
Title: Perioperative Nivolumab in Resectable Lung Cancer
Authors: Tina Cascone, Mark M Awad, Jonathan D Spicer, Jie He, Shun Lu, Boris Sepesi, Fumihiro Tanaka, Janis M Taube, Robin Cornelissen, Libor Havel, Nina Karaseva, Jaroslaw Kuzdzal, Lubos B Petruzelka, Lin Wu, Jean-Louis Pujol, Hiroyuki Ito, Tudor-Eliade Ciuleanu, Ludmila de Oliveira Muniz Koch, Annelies Janssens, Aurelia Alexandru, Sabine Bohnet, Fedor V Moiseyenko, Yang Gao, Yasutaka Watanabe, Cinthya Coronado Erdmann, Padma Sathyanarayana, Stephanie Meadows-Shropshire, Steven I Blum and Mariano Provencio Pulla.

Source: Toni Choueiri /X
Toni K. Choueiri is the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute (DFCI), co-leader of the Kidney Cancer Program at Dana-Farber/Harvard Cancer Center, and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School. As a medical oncologist, clinical trialist, and translational researcher, he specializes in treating genitourinary cancers (prostate, bladder, testis, and kidney cancer), with a focus on kidney cancer.


