Sumanta K. Pal shared a post on X:
“Huge congrats to Pedro C Barata, who drove this important analysis of SWOG Cancer Research Network 1500, published just ahead of ESMO24 in Journal of Clinical Oncology. Lots of dialogue here in Barcelona around management of non-clear kidney cancer; I think this paper & data from SUNNIFORECAST are important parts of the dialogue.
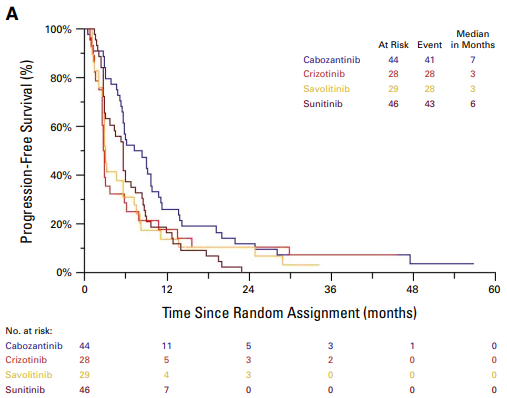
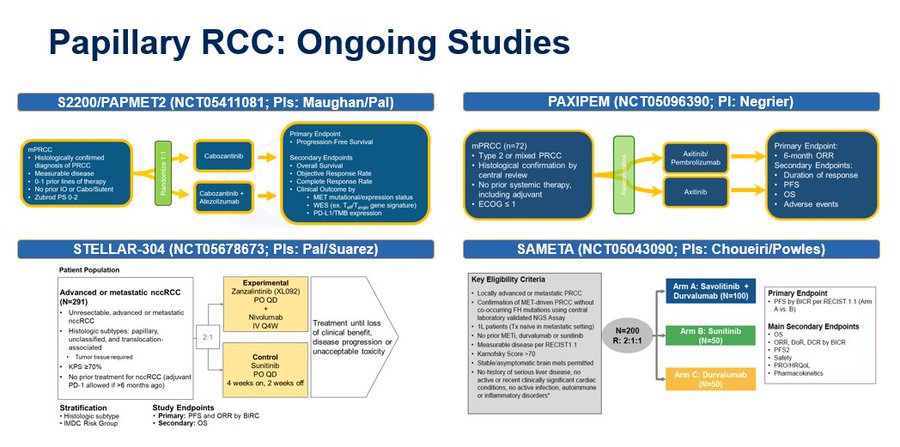
In this final analysis of OS from S1500, we show no OS advantage with cabozantinib over sunitinib. PFS & OS curves are shown below. The PAPMET data have inspired PAPMET2, led by Benjamin L Maughan, Huntsman Cancer Institute, which I will discuss shortly.
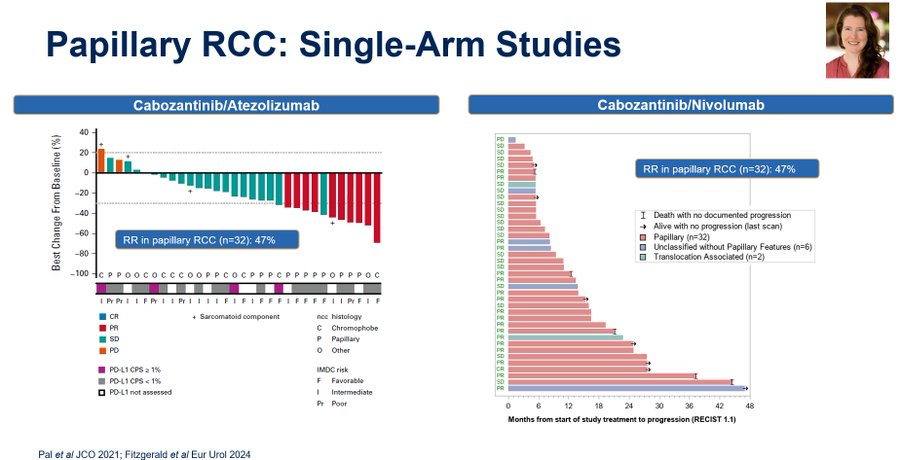
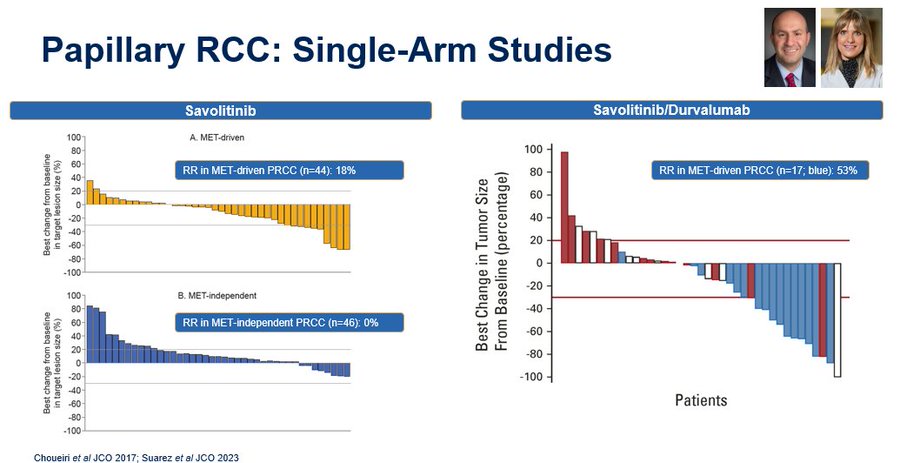
So what is the current standard for nonclear cell kidneycancer, and perhaps more specifically papillary? SWOG Cancer Research Network 1500 showed that we can do randomized studies in this space, but many of our dialogues today are fueled by single arm experiences. Neeraj Agarwal, Bradley McGregor & I (along with others) have looked at cabozantinib with atezolizumab, and Kelly Fitzgerald recently updated data for cabozantinib with nivolumab in European Urology. Impressive RRs & both with a RR in papillary of 47%.
There are other VEGF-IO studies.
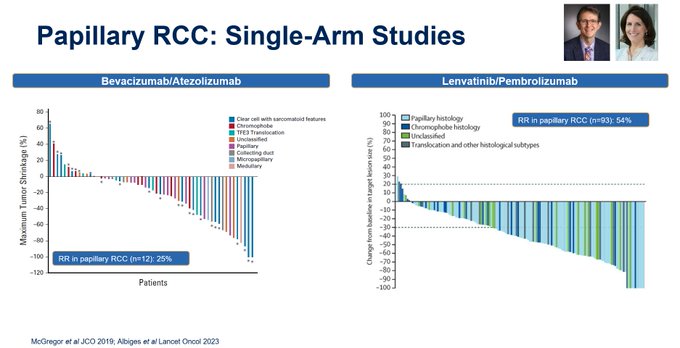
Bradley McGregor has published the data for bevacizumab w atezolizumab (more modest RRs) and Laurence Albiges has published data for lenvatinib w pembrolizumab in KEYNOTEB61. But do these single arm studies reflect SOC?
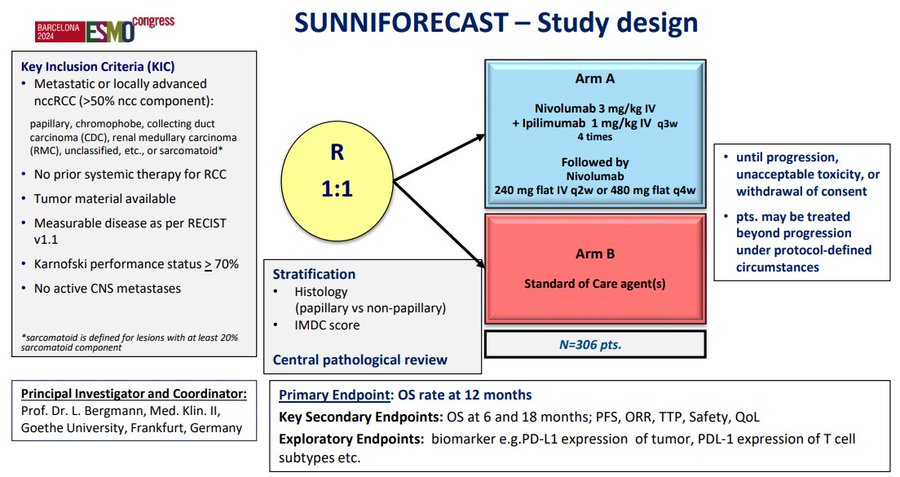
In my mind, definitely not. We have proven that we CAN do randomized studies in papillary kidney cancer, so we MUST do them to ensure we are not (a) offering additional therapy without CONFIRMED advantage and (b) not causing undue harm. Now onto SUNNIFORECAST from ESMO24.
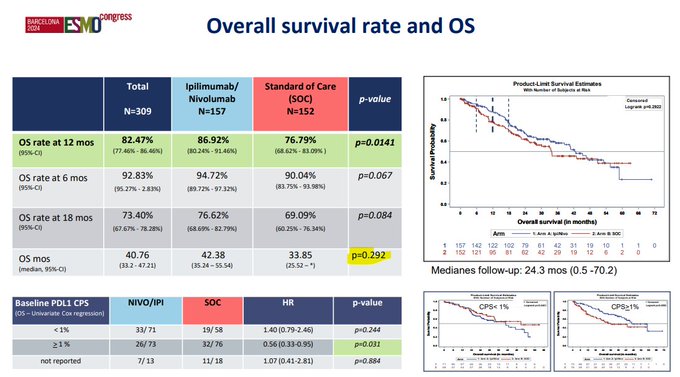
While I applaud the investigators for conducting a randomized study in this setting, I think we have to concede that the primary endpoint (1-year OS) is unconventional. Looking at the data in amalgam, there is no difference in PFS or OS with nivolumab & iplimumab.
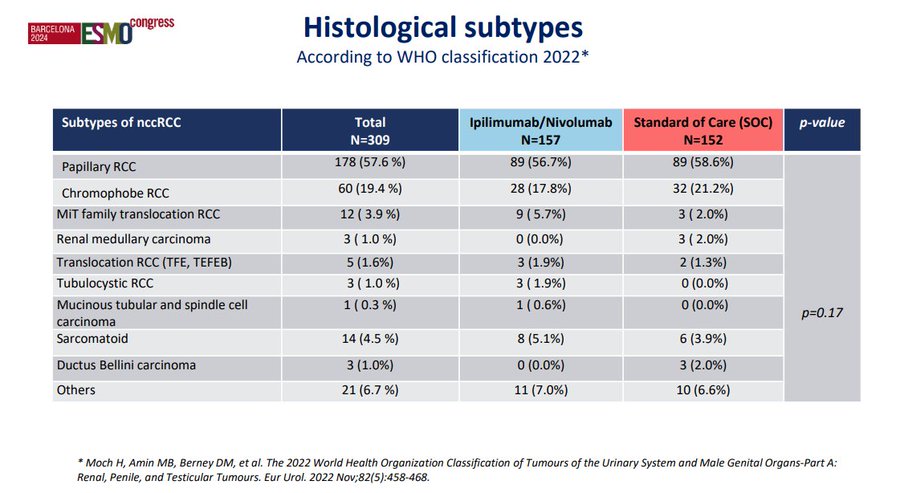
And while there were some groups of interest (e.g., high PDL1 expressors), this was a VERY heterogeneous group of patients. Could the smattering of pts with sarcomatoid features have driven outcomes? Were there pts where these features were unrecognized? And what was the contribution of the exquisitely rare subtypes which likely carry a completely unique biology?
I definitely don’t think nivolumab/ipilimumab moves into the category of established standard. As a community, I think we need renewed focus on some of the randomized studies that are currently ongoing which may more definitively establish the role of doublet therapies. We also need to understand the role of biological selection – e.g., in papillary kidney cancer, should we be using MET status to allocate therapy? Toni Choueiri, Suarez, Tom Powles et al have contributed greatly to our understanding of savoltinib alone & (in single arm studies) combinatino with durvalumab.
So what are those randomized trials? I’ve summarized them here. PAPMET2, STELLAR304, SAMETA & several others require our attention as a community. It’s imperative that we definitively establish the role of TKI/IO regimens for papillary kidney cancer & other nonclear cell types.”
Authors: Pedro C Barata, et al.

Source: Sumanta K. Pal/X
Sumanta (Monty) Pal, MD, is a medical oncologist and an expert in genitourinary cancers at the City of Hope Comprehensive Cancer Center in Los Angeles. He serves as the Vice Chair of Academic Affairs, Professor and co-director of the Kidney Cancer Program and leads the Kidney and Bladder Cancer Disease Team at City of Hope. He specialises in kidney, bladder, and prostate cancers.