Kai Rejeski shared a post on X about a recent paper by Tobias Tix et al. published in Clinical Cancer Research.
Authors: Tobias Tix, Mohammad Alhomoud, Roni Shouval, Edward Scheffer Cliff, Miguel-Angel Perales, David Cordas dos Santos, Kai Rejeski

“Excited to share our new paper focusing on Second Primary Malignancies (SPMs) following CAR T-cell therapy out today in Clinical Cancer Research!
This first-in-kind meta-analysis spans 5,517 lymphoma and myeloma patients receiving a range of CAR-T products.
Post CAR-T SPMs (and particularly T cell malignancies) garnered significant media coverage following the FDAs class-wide blackbox warning End of 2023.
Initial studies using the FAERS database had indicated increased reporting of SPM but are limited due to reporting bias.
In terms of topline results, we found an overall SPM estimate of 5.8% at about 2 years of follow up.
While we noted minor variations by product or disease indication, none of these differences reached statistical significance.
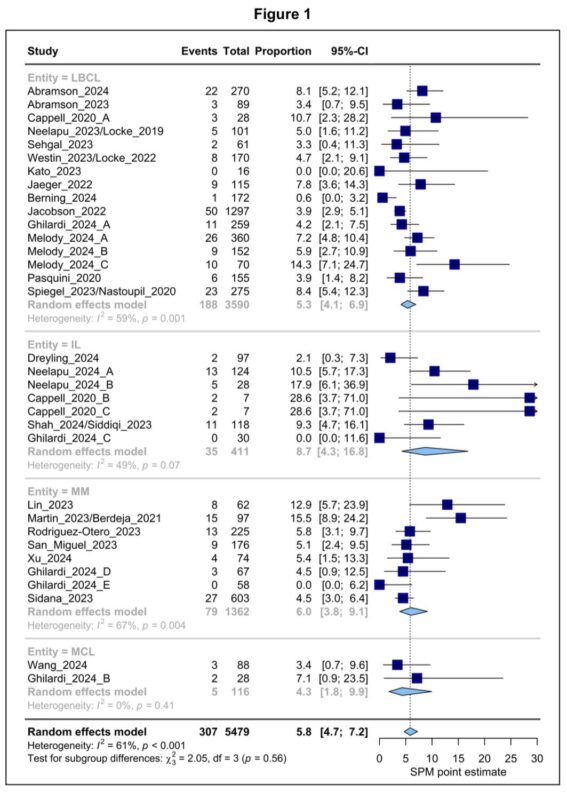
On metaregression modelling, we found that SPM occurrence after CART is associated with:
- duration of follow up
- number of prior therapy lines
- treatment setting (CT > RWS)
I’ll note that none of these study-level factors are intrinsically linked to CAR-T therapy itself.
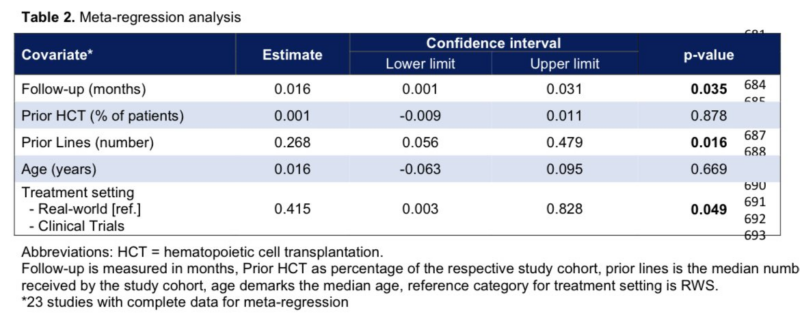
In a subgroup meta analysis of the randomized Ph 3 clinical trials, we did not identify an increased frequency of SPMs with CAR-T relative to previous standard-of-care therapies
Examples:
- auto-HCT for lymphoma
- IMID-based tx for myeloma
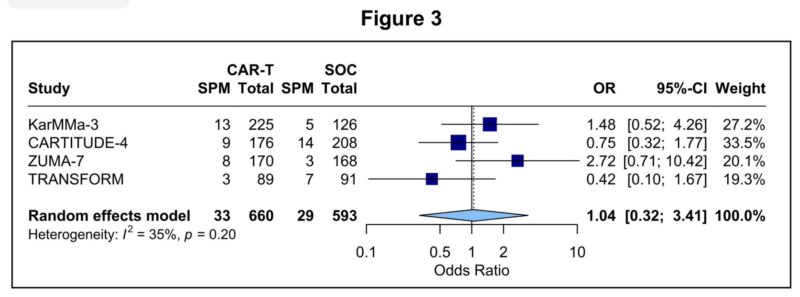
Finally the breakdown of the 326 SPM cases shows that the majority of SPMs are heme malignancies followed by solid tumors.
T cell malignancies were exceedingly rare (point estimate of 0.09%) and not all of these were CAR +
Even in the handful of reported CAR+ lymphoma cases, in-depth tissue analyses have questioned to what extent insertional mutagenesis actually contributed to TCM development.
Great work from Guido Ghilardi, Marco Ruella, Ash Alizadeh and others in this space!
Future research should focus on better understanding the true mechanistic link between CAR-T therapy and SPM development, recognizing that the pathogenesis is likely to be multifactorial.
What is the role of:
- Prior tumorigenic therapies
- Lymphodepletion
- Presence of CHiP?
- CAR-T mediated inflammation?
- Immune suppression (eg, decreased immune surveillance)
Much to be learned here!
It is also important to stress that you need to be alive to develop a SPM, highlighting the role of survivorship in CAR-T recipients.
Something that Michael Jain, Jay Spiegel and Saurabh Dahiya eloquently stressed in their recent JCO paper.
Ultimately, this meta-analysis questions the FDAs class-wide blackbox warning. Our results do not indicate that the risk is higher with CAR-T relative to alternative treatment strategies and that exposure to previous lines of therapy and extended FU both play a critical role.
Nonetheless, while we are still learning about long term adverse events of CART (incl SPM and late infections), it is all about: reporting, reporting, reporting….as hopefully the basis for improved understanding of long term CAR-T toxicities.
Big thanks to the entire team Memorial Sloan Kettering Cancer Center and particularly Mohammad Alhomoud, Roni Shouval and Miguel Perales for their support with this project!
Also want to give a particular shout-out to rockstar resident from LMU Clinic, who did a fantastic job as the lead author on this effort!
And of course Eddie Cliff as well as my partner in crime David Cordas dos Santos from Dana-Farber Cancer Institute.”
Source: Kai Rejeski/X
More posts featuring Kai Rejeski on oncodaily.com
Kai Rejeski is a Visiting Investigator and Research Fellow at Memorial Sloan Kettering Cancer Center, as well as a Resident Physician and Clinical Fellow at LMU München Clinic. His research focuses on mapping and modeling CAR T-cell toxicities and using AI-based morphometric techniques to enhance clinical decision-making and improve the safety of cellular therapies.


