Toni Choueiri shared a post on X about a recent paper titled “Belzutifan versus Everolimus for Advanced Renal-Cell Carcinoma” published in New England Journal of Medicine (NEJM).
Authors: Toni Choueiri, Thomas Powles, Katriina Peltola, Laurence Albiges, Brian Rini et al.
“Results from LITESPARK-005 are out in NEJM!
The phase 3 trial that led to HIF-2 inhibitor Belzutifan (BEL) U.S. FDA approval Dec 2023 in pretreated patients with ccRCC.
Presentation ESMO24.
Alterations in VHL are seen in >90% of ccRCC VHL negatively regulates the HIF pathway (NobelPrize 2019, William Kaelin’s lab) targeting HIF2a with belzutifan (MK-6482) showed promising results in early trial, published Nature Medicine (link).
Review of this story also in Nature Medicine with William Kaelin’s lab.
LITESPARK-005 is a phase 3, multicenter, open-label trial
Inclusion criteria were:
- Metastatic ccRCC
- KPS >= 70
- Prior progression on 1-3 regimens including anti-PD(L)1 AND VEGF TKI.
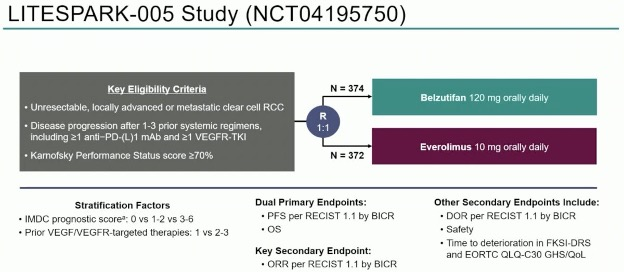
Patients were randomized 1:1 to receive BEL 120mg/d or Everolimus (EVE) 10mg/d.
Dual 1ary endpoints were PFS (central review) and OS.
2ary endpoint was ORR (central review).
746 patients were enrolled in the ITT: 374 belzutifan vs. 372 everolimus.
Time from randomization to data cutoff for the 1st and 2nd interim analyses was 18.4 and 25.7 months resp. Baseline characteristics:
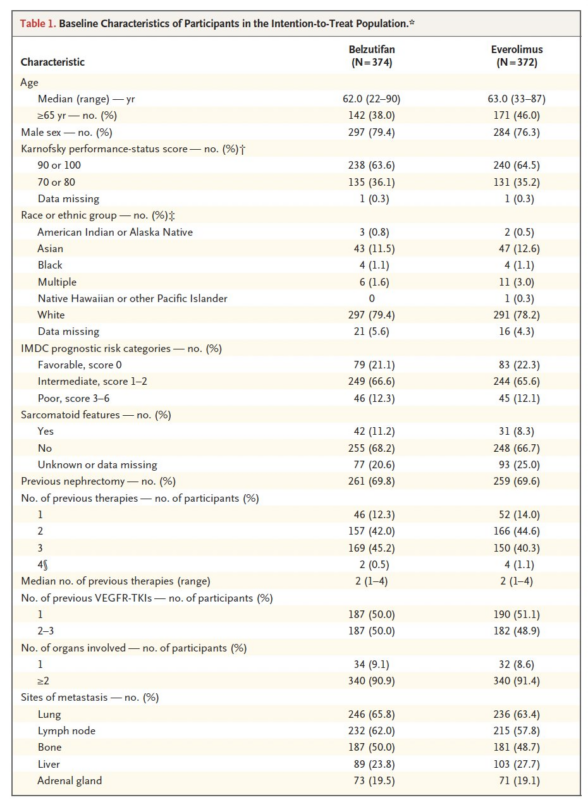
A PFS benefit was observed with BEL vs EVE.
At 18 months, 24% of patients in the BEL group vs. 8.3% in the everolimus group were alive and free of progression (P = 0.002)
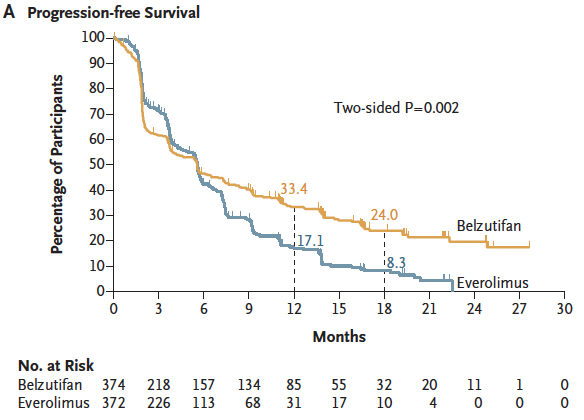
Similarly, an ORR benefit was also observed with BEL vs EVE At 25 months, ORR 22.7% with belzutifan vs. 3.5% with everolimus.
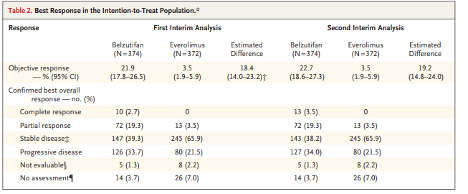
Median OS was 21.4 months in the BEL group vs. 18.1 months in the EVE group (HR 0.88; 95% CI, 0.73 to 1.07; P = 0.20).
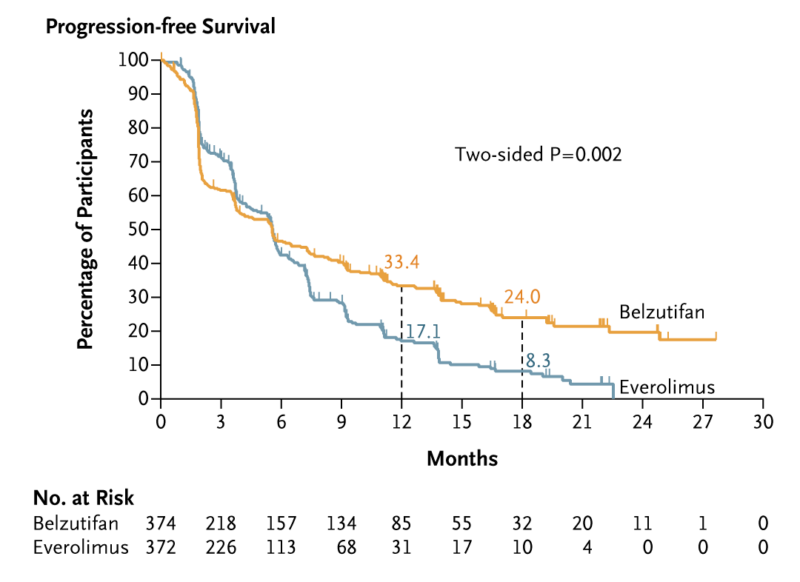
Grade 3+ adverse events were ~62% in both treatment arms.
Most common AEs with belzutifan were anemia and hypoxia.
AEs led to discontinuation of treatment in 5.9% and 14.7% of patients on BEL and EVE, respectively.
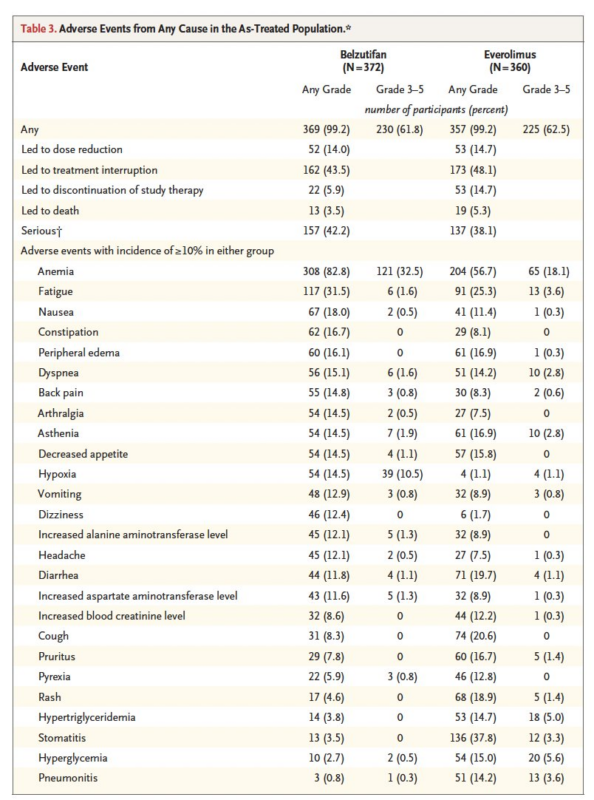
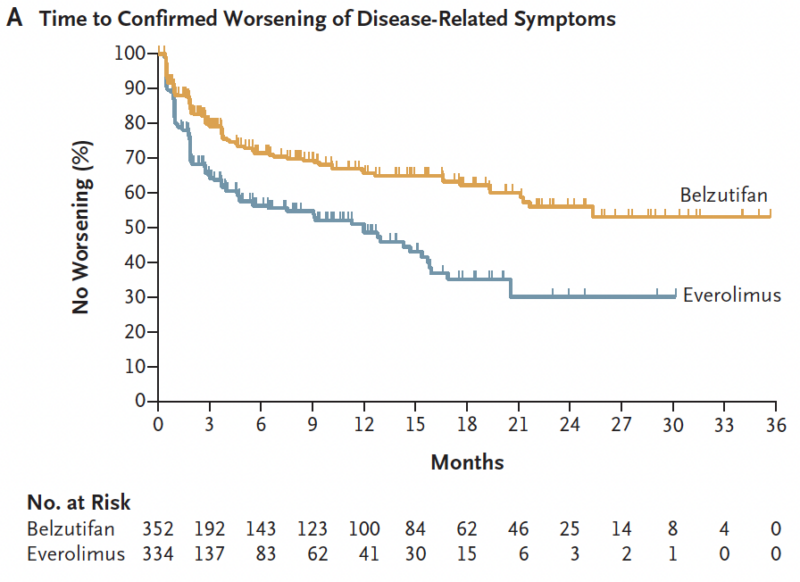
Finally, BEL significantly prolonged the time to worsening of disease-related symptoms (HR 0.53, 95%CI 0.41-0.69) and the time to deterioration of QoL (HR 0.75, 95% CI 0.58-0.96) compared to EVE.
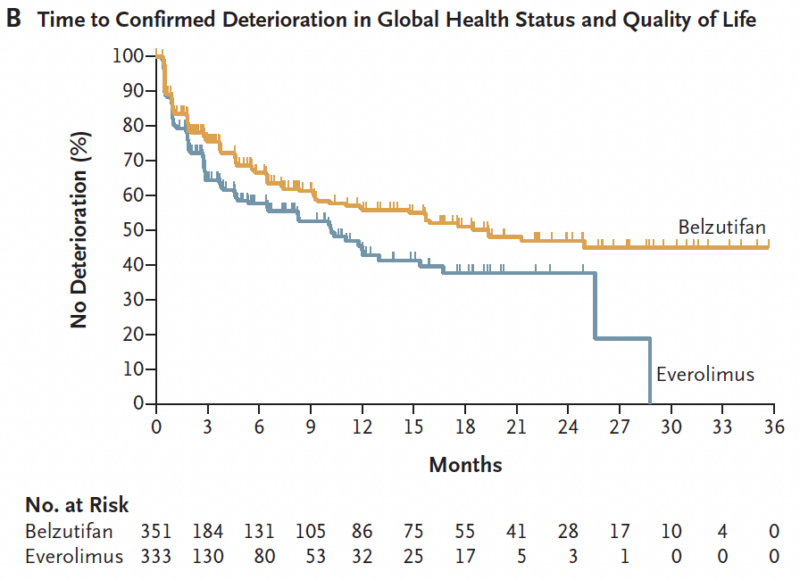
In conclusion, the HIF2a inhibitor belzutifan led to a PFS and ORR benefit vs. everolimus in patients with metastatic ccRCC having progressed on prior ICI and VEGF TKI.
Huge thanks to all the investigators who made this trial possible, to Merck, the sponsor, and mostly to our patients and their families, to whom we dedicate all our efforts!
Amazing and gratifying personal journey with HIF2, from Bill’s (and many others) work, to the 1st generation compound (PT2385) to PT2977->MK-6482-> BEL.
“Phase I Dose-Escalation Trial of PT2385, a First-in-Class Hypoxia-Inducible Factor-2α Antagonist in Patients With Previously Treated Advanced Clear Cell Renal Cell Carcinoma” with Kevin Courtney/James Brugarolas
Over a decade with Dana-Farber/Harvard Cancer Center (DF/HCC) Kidney SPORE projects, U01, and integrating BEL into adjuvant/1L/2L trials (all completed). The Journey continues!”

Colleagues and other oncologists made their comments regarding this article:
NEJM:
“In participants with advanced renal-cell carcinoma, belzutifan, a HIF-2α inhibitor, was superior to everolimus, an mTOR inhibitor, with respect to progression-free survival and tumor response. Read the full LITESPARK-005 trial results.”
“Huge congrats Toni Choueiri, Brian Rini, Tom Powles, Laurence Albiges on this tremendous work advancing the care of kidney cancer patients.”
“Wowza! Huge congratulations to Toni Choueiri et al. on the fantastic NEJM publication!
Belzutifan showed a significant benefit over everolimus – PFS and ORR in participants with advanced clear-cell renal-cell carcinoma s/p Immunotherapy and anti-VEGF Tx!”
“Huge congrats Toni Choueiri! You continue to set the standard in kidney cancer!”
“Congrats Toni Choueiri, Tom Powles, Laurence Albiges, Brian Rini, Guillermo de Velasco et al. for a practice-changing trial! ESMO, NEJM important milestone for advanced RCC!”
More posts by Toni Choueiri on oncodaily.com
Toni Choueiri is the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute (DFCI), co-leader of the Kidney Cancer Program at Dana-Farber/Harvard Cancer Center, and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School. He is the Medical Director of International Strategic Initiatives at Dana-Farber and past President of the Medical Staff at DFCI (2016-2018).
Shilpa Gupta is the Director of Genitourinary Oncology, a staff member in Hematology and Oncology and a Professor of Medicine at Cleveland Clinic. Dr. Gupta’s extensive research focuses on novel drug development and biomarkers of response/resistance in bladder cancer, contributing to numerous published works and leadership roles in clinical trials.
Vivek Subbiah is the Chief of Early-Phase Drug Development at the Sarah Cannon Research Institute (USA). He is the former Executive Director of Oncology Research and former Associate Professor in the Department of Investigational Cancer Therapeutics at the MD Anderson Cancer. He focuses on translational cancer research and the design and implementation of early-phase biomarker-driven clinical trials. His work specifically targets antibody-drug conjugates, radiopharmaceuticals, immunoconjugates, and basket trials.
Sumanta (Monty) Pal, MD, is a medical oncologist and an expert in genitourinary cancers at the City of Hope Comprehensive Cancer Center in Los Angeles. He serves as the Vice Chair of Academic Affairs, Professor and co-director of the Kidney Cancer Program and leads the Kidney and Bladder Cancer Disease Team at City of Hope. He specialises in kidney, bladder, and prostate cancers.
Petros Grivas, a board-certified medical oncologist, serves as Clinical Director of the Genitourinary Cancers Program at the University of Washington and Seattle Cancer Care Alliance. He’s an Associate Professor at the Dept. of Medicine and Associate Member at Fred Hutchinson Cancer Research Center since January 2018. With extensive training and experience, he’s led clinical trials, contributing to FDA approvals for bladder/urothelial cancer treatments.


