Grainne O’Kane, Medical Oncologist at Princess Margaret Cancer Centre, shared a post by Kenneth Olive, Co-Leader of the Precision Oncology and Systems Biology Program at Columbia University Irving Medical Center, adding:
“Such an exciting time! Likely game-changer in PDAC KRASi
- hopefully we can understand who will respond, who will need combinations
- how will subtypes and RAS dosage influence response?
- how can we help more patients access trials!”
Quoting Kenneth Olive’s post:
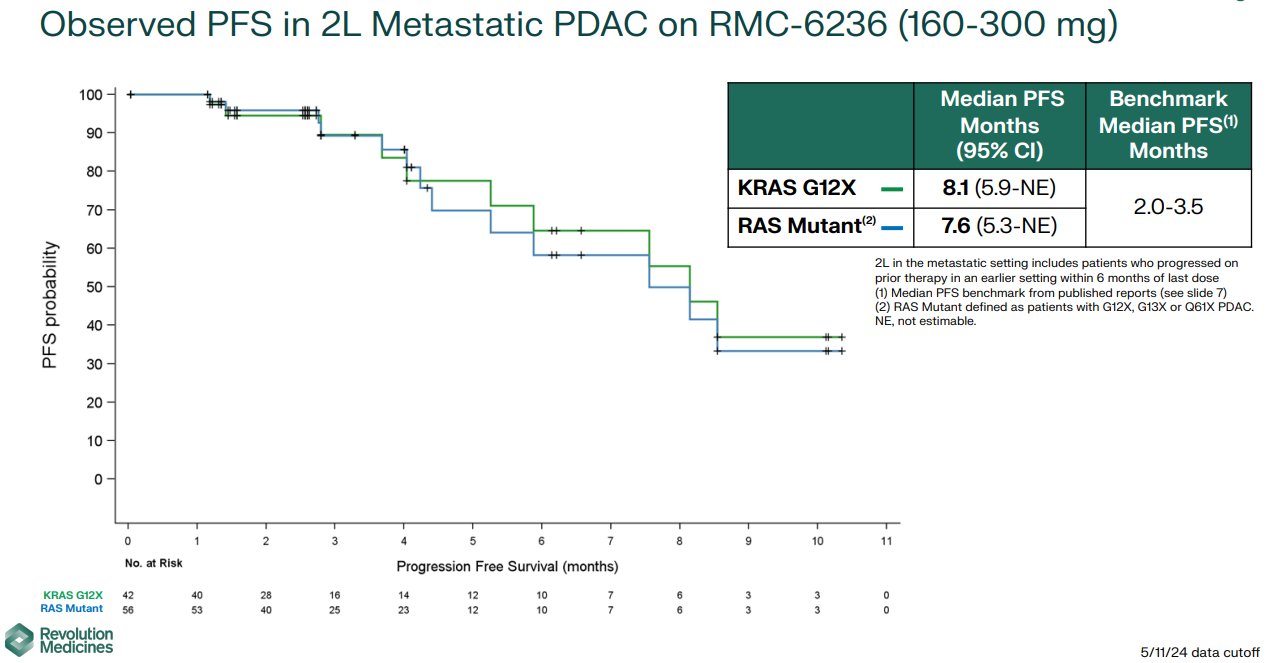
“Stunning interim clinical trial data from Revolution Medicines… PFS = 8.1 mo in 2nd line KRAS G12-mutant metastatic PDAC. OS not yet reached, but already more than a year! Benchmark PFS for this group is 2-3.5 months, behnchmark OS is 6.1-6.9.
All standard caveats apply: single arm data, non randomized, highly scrutinized patients, early phase, selected group, etc. Even with the caveats, the results are exciting IMHO.
They present a preliminary design of a planned Phase 3 trial in 2nd line PDAC patients, randomized to physician’s choice.
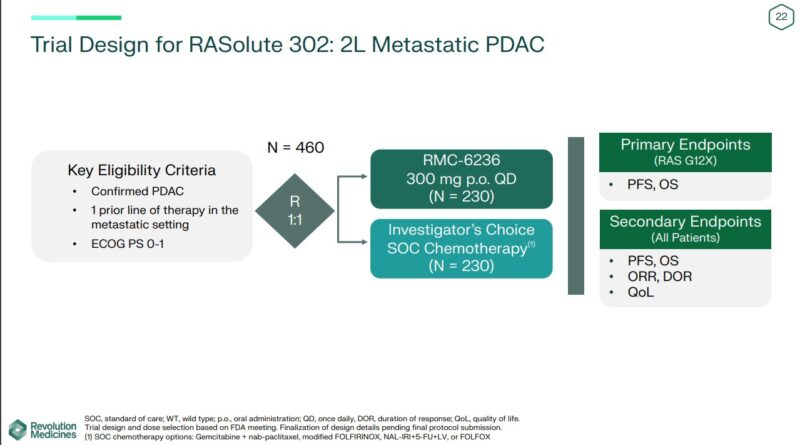
Data available on their website.
Really exciting times for the PDAC field, offering real hope for patients. This is just monotherapy. Can’t wait to see combinations!”
Sources: Kenneth Olive/X and Grainne O’Kane/X