PopEVE, According to mtsoln.com, is a new artificial intelligence model redefining how clinicians diagnose rare diseases—offering faster insights, higher accuracy, and a transformative leap in patient care.
AI Breakthrough Targets the Rare Disease “Diagnostic Odyssey”
Diagnosing a rare disease is often a long, exhausting journey. Many patients spend years moving between doctors, hospitals, and tests before anyone can attach a name to their condition. With more than 10,000 known rare diseases and huge overlap in symptoms, even experienced clinicians can struggle to find the right answer.
PopEVE, the new AI model described by mtsoln.com, has been designed specifically to address this challenge. It analyzes complex clinical data at scale and supports clinicians in narrowing down difficult diagnoses more quickly and more accurately than traditional approaches.
Instead of relying solely on individual specialist experience, PopEVE brings together massive amounts of medical information and pattern recognition into a single, intelligent system.
Evidence Behind PopEVE: What the New Study Actually Shows
Based on the latest article published by Orenbuch and Colleagues in Nature Genetics analysis involving data from 31,000+ rare disease trios, researchers demonstrated that the model can identify highly deleterious missense variants with precision far beyond current tools. The original PopEVE study showed that integrating deep evolutionary patterns with human population constraint allows the model to calibrate pathogenicity scores across the entire proteome — something no previous system was able to do reliably.
This matters clinically because rare diseases are often driven by never-before-seen variants, and traditional models are accurate only in previously annotated genes. PopEVE overcomes this by learning from two massive data sources simultaneously:
- Evolutionary conservation across billions of years of divergence
- Human population variation from UK Biobank and gnomAD
By fusing these signals, PopEVE generates a single deleteriousness score that a clinician can interpret consistently — whether the mutation is in a well-known disease gene or a completely uncharacterized one.
What the Data Shows?
The model was tested using >30,000 de novo missense mutations from patients with severe developmental disorders. PopEVE demonstrated:
- 15-fold enrichment for highly deleterious variants in affected individuals compared with controls
- Ability to identify 123 previously unknown candidate developmental disorder genes
- >90% of top-scoring variants located near known functional or structural protein interaction sites
- 98% accuracy in ranking likely causal variants above all inherited variants in the same patient
- Only 0.2% of healthy controls carried variants with equivalently severe scores
For oncologists and pharma teams, the key takeaway is that the model does not inflate pathogenicity — a common problem with older variant prediction tools. In real-world genetic datasets, PopEVE predicted far fewer false-positive “pathogenic” variants than AlphaMissense, REVEL, or BayesDel, while still recalling more true disease-causing variants.
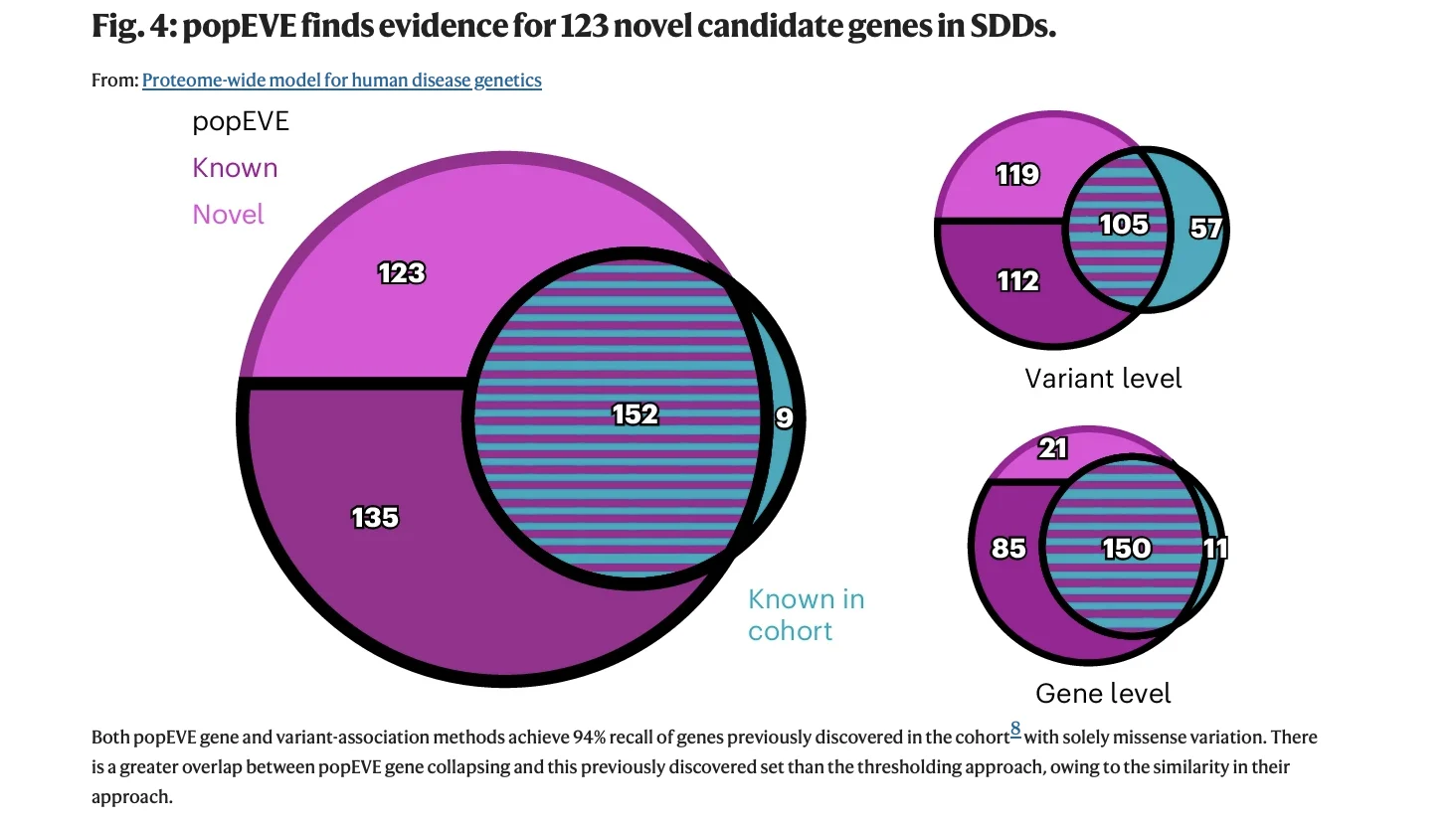
Source: Orenbuch et al, Proteome-wide model for human disease genetics, Published in Nature Genetics
From Scattered Clues to Clearer Diagnostic Paths
One of the hardest parts of diagnosing rare diseases is that patients rarely present with a single striking hallmark symptom. More often, they have a mixture of seemingly unrelated problems: fatigue, unexplained pain, odd lab abnormalities, neurologic signs, or immune disturbances that don’t quite fit common patterns.
PopEVE is designed to bring order to this chaos. It reviews the patient record as a whole—symptom timelines, lab trends, imaging reports, genetic information when available—and looks for hidden structure in the data. Where a human might see a confusing mix of findings, the model can detect recurring combinations that match known rare diseases.
Instead of offering one rigid answer, PopEVE returns a set of possible diagnoses with likelihood estimates. This gives clinicians a focused shortlist to explore with targeted tests, consultations, or genetic workups, rather than starting from scratch or ordering broad, costly panels.
Supporting Clinicians, Not Replacing Them
Importantly, PopEVE is not meant to replace doctors. It is a decision-support tool that works alongside clinicians, elevating their ability to recognize rare conditions early.
In practice, this means the AI can flag high-risk cases for closer review, suggest diseases that may not have been initially considered, and highlight patterns that merit further investigation. The final diagnosis still depends on the physician’s clinical judgment, examination, and confirmatory testing.
This human–AI partnership is especially powerful in settings where specialists in rare diseases are scarce. A generalist in a community hospital can benefit from the same advanced analytical support as a doctor in a major academic center, reducing disparities in access to expert-level diagnostics.
Transforming Pediatric and Genetic Diagnostics
The potential impact of PopEVE is particularly strong in pediatrics and genetics, where rare diseases are common and early diagnosis can be life-changing.
Many childhood-onset rare disorders have a genetic basis, but their first signs can be subtle—delayed development, unusual growth patterns, unexplained seizures, or recurrent infections. In such cases, PopEVE can help clinicians recognize when a pattern looks more like a rare syndrome than a familiar common illness.
By narrowing the list of suspected conditions, the model can guide more efficient use of genetic testing and specialist referrals. Families may reach a diagnosis months or years earlier than they otherwise would, making it easier to start the right treatments, plan long-term care, and connect with appropriate support networks.

Read More About Rare Cancer Day on OncoDaily
Closing the Gap Between Regions and Health Systems
Rare disease expertise is unevenly distributed worldwide. Large academic hospitals may have dedicated centers and specialists, but many regions and smaller facilities do not. Patients in rural or low-resource settings often face longer delays and fewer specialist options.
A model like PopEVE can help bridge that gap. Because the AI can be integrated into electronic health record systems or clinical decision platforms, its insights can be made available nearly anywhere with digital infrastructure. The same advanced diagnostic support that informs a major urban teaching hospital can, in principle, be extended to clinics hundreds of kilometers away.
This has major implications for health equity: earlier suspicion of rare disease, more appropriate referrals, and fewer patients left undiagnosed simply because of where they live.
Safeguards, Transparency, and Trust
As with any medical AI system, trust and safety are central to PopEVE’s design. The model is intended to be transparent enough that clinicians can understand why it suggested certain diseases. This interpretability is key to building confidence and avoiding blind reliance on algorithmic output.
Patient privacy and data security are also critical. Systems like PopEVE must follow strict standards to protect sensitive health information, while still learning from aggregated, anonymized data to improve performance over time.
Ultimately, PopEVE is framed not as an “auto-diagnosis machine,” but as an advanced, learning companion to healthcare professionals—one that amplifies human expertise rather than replacing it.
Toward a Future Where Rare Diseases Are Found Sooner
The emergence of PopEVE, as reported by mtsoln.com, signals a meaningful shift in how medicine tackles rare diseases. Instead of accepting long delays as inevitable, health systems now have tools that can turn scattered clues into earlier, more accurate diagnoses.
If successfully deployed at scale, PopEVE could shorten the diagnostic odyssey for many patients, reduce unnecessary testing and referrals, and support clinicians facing increasingly complex caseloads. For individuals and families living with rare conditions, that doesn’t just mean a faster label—it can mean access to the right treatments, better symptom management, and a clearer path forward.
In a field where every month without an answer can feel like an eternity, an AI model like PopEVE represents something powerful: the possibility that rare diseases become visible, recognized, and addressed far sooner than ever before.


