Small cell lung cancer (SCLC) is an aggressive malignancy, with most patients diagnosed at the extensive stage (ES-SCLC). While the addition of atezolizumab to platinum-etoposide chemotherapy established immunotherapy-based first-line treatment, relapse remains common. Maintenance strategies that prolong survival are urgently needed.
Lurbinectedin (Zepzelca) is a selective inhibitor of oncogenic transcription programs with established activity in relapsed SCLC. On October 2, 2025, the FDA approved lurbinectedin in combination with atezolizumab (IV) or atezolizumab and hyaluronidase-tqjs (subcutaneous) for maintenance treatment of adult patients with ES-SCLC whose disease has not progressed after induction with atezolizumab ± hyaluronidase-tqjs, carboplatin, and etoposide.
IMforte Trial
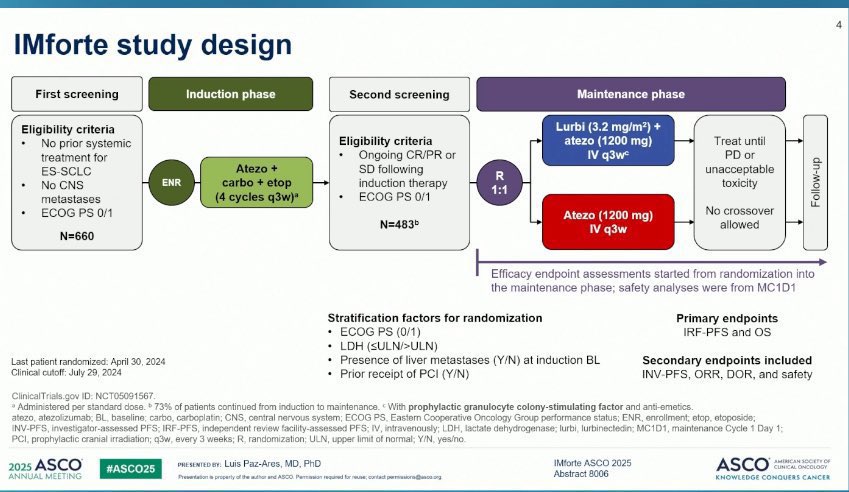
The IMforte trial (NCT05091567) was a randomized, multicenter, open-label phase III study designed to evaluate the role of lurbinectedin in combination with atezolizumab as maintenance therapy for extensive-stage small cell lung cancer. The study enrolled 483 patients whose disease had not progressed after completing four cycles of induction therapy with atezolizumab, carboplatin, and etoposide. Following induction, patients were randomized in a 1:1 ratio to receive either lurbinectedin plus atezolizumab intravenously as maintenance treatment or atezolizumab alone. The major efficacy endpoints were overall survival and progression-free survival, both assessed from the time of randomization after induction therapy.
Efficacy Results
Overall Survival (OS):
- Lurbinectedin + Atezolizumab: 13.2 months (95% CI 11.9–16.4)
- Atezolizumab alone: 10.6 months (95% CI 9.5–12.2)
- HR 0.73 (95% CI 0.57–0.95); p=0.0174
Progression-Free Survival (PFS):
- Lurbinectedin + Atezolizumab: 5.4 months (95% CI 4.2–5.8)
- Atezolizumab alone: 2.1 months (95% CI 1.6–2.7)
- HR 0.54 (95% CI 0.43–0.67); p<0.0001
Safety Profile
The safety profile of the combination was consistent with what is already known for both agents.Lurbinectedin carries warnings for myelosuppression, hepatotoxicity, extravasation that can lead to tissue necrosis, rhabdomyolysis, and embryo-fetal toxicity.
Atezolizumab, whether given intravenously or with hyaluronidase, is associated with risks of severe or even fatal immune-mediated adverse events, infusion-related reactions, complications following allogeneic stem cell transplantation, and embryo-fetal toxicity. Importantly, in the IMforte trial, adverse events aligned with these established safety profiles, and no new or unexpected toxicities emerged.

Recommended Dosage
- Lurbinectedin: 3.2 mg/m² IV every 21 days until progression or unacceptable toxicity.
- Atezolizumab (IV): 840 mg q2w, 1200 mg q3w, or 1680 mg q4w.
- Atezolizumab + Hyaluronidase-tqjs (SC): 1875 mg atezolizumab + 30,000 units hyaluronidase every 3 weeks