What Does FDA Approval Mean?
The U.S. Food and Drug Administration (FDA) evaluates new cancer therapies to determine whether they are safe and effective for clinical use. Approval is based on rigorous review of clinical trial data, with the goal of making evidence-based treatments available to patients. When a therapy receives FDA approval, it becomes an accepted option in routine practice, often changing the standard of care.
In oncology, approvals may also come through expedited programs such as priority review, breakthrough designation, and Project Orbis, which accelerate regulatory timelines for serious diseases with unmet needs—as occurred in this case.
Standard Treatment for Muscle-Invasive Bladder Cancer
Muscle-invasive bladder cancer (MIBC) is an aggressive disease characterized by invasion of the detrusor muscle (≥T2). The historical standard of care consists of:
• Neoadjuvant cisplatin-based chemotherapy, followed by
• Radical cystectomy (RC) with pelvic lymph node dissection (PLND).
However, 40–50% of patients are cisplatin-ineligible due to renal insufficiency, neuropathy, hearing loss, frailty, or comorbidities. For these patients, no effective perioperative systemic therapy has been available; many proceed directly to surgery, where outcomes remain suboptimal.
Enfortumab vedotin (EV), an antibody–drug conjugate targeting Nectin-4, combined with pembrolizumab (anti–PD-1), has demonstrated substantial activity in advanced and metastatic urothelial cancer. This served as the mechanistic rationale to evaluate the combination earlier in the curative-intent setting.
KEYNOTE-905/EV-303 Trial
Cisplatin-based chemotherapy is the standard neoadjuvant treatment for muscle-invasive bladder cancer, but nearly half of patients cannot receive cisplatin because of comorbidities such as renal insufficiency, neuropathy, or frailty. These patients traditionally proceed directly to radical cystectomy, where outcomes remain poor.
KEYNOTE-905/EV-303 was therefore developed to determine whether a perioperative regimen combining enfortumab vedotin and pembrolizumab could improve survival outcomes compared with surgery alone in this high-risk, cisplatin-ineligible population.
Study Design and Methods
KEYNOTE-905/EV-303 is a phase 3, randomized, open-label, multicenter trial enrolling adults with previously untreated, resectable muscle-invasive bladder cancer. Eligible patients had:
- MIBC classified as T2–T4aN0M0 or T1–T4aN1M0
- No prior systemic therapy for bladder cancer
- Ineligibility for cisplatin based on Galsky criteria, or personal refusal of cisplatin despite being eligible
Participants were randomized 1:1 to receive either perioperative systemic therapy with surgery or surgery alone.
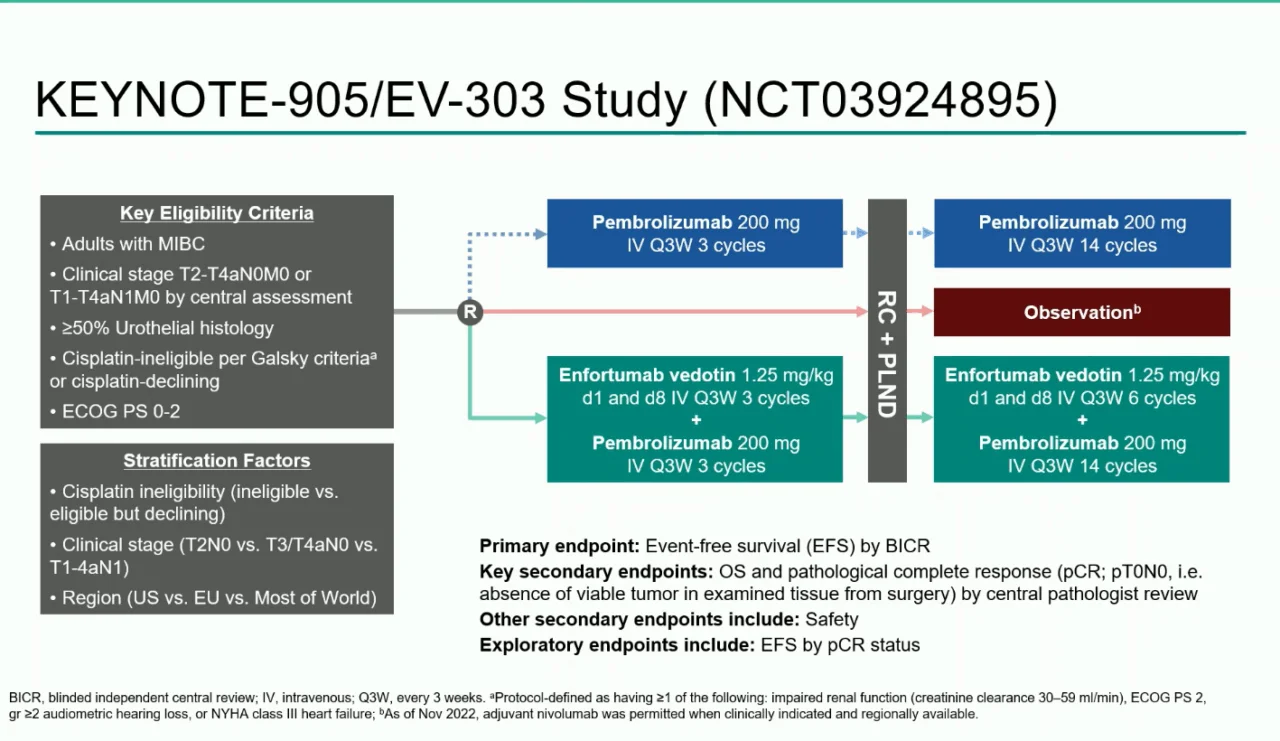
Treatment Arms
The trial compared perioperative enfortumab vedotin plus pembrolizumab with standard surgery alone.
Enfortumab Vedotin + Pembrolizumab Arm (EV + Pembrolizumab)
Patients assigned to the investigational arm received a structured perioperative treatment plan. In the neoadjuvant phase, before surgery, they were treated with enfortumab vedotin at a dose of 1.25 mg/kg on days 1 and 8 of a 21-day cycle, together with pembrolizumab 200 mg every three weeks. This combination was administered for three full cycles before patients proceeded to radical cystectomy with pelvic lymph node dissection (RC + PLND).
Following surgery, patients entered the adjuvant phase, where therapy continued with six additional cycles of enfortumab vedotin. Pembrolizumab was also maintained postoperatively, given either every three weeks for up to 14 cycles or every six weeks for up to seven cycles, depending on the chosen schedule. After completing the combination phase, patients continued pembrolizumab monotherapy to complete the intended duration of adjuvant immunotherapy.
Control Arm
Patients assigned to the control arm underwent immediate radical cystectomy with pelvic lymph node dissectionwithout any planned neoadjuvant therapy. Postoperatively, adjuvant nivolumab was permitted at the discretion of the treating physician and local practice guidelines, reflecting real-world standards of care for high-risk patients after surgery.
Endpoints
The primary endpoint was event-free survival (EFS) as assessed by blinded independent central review.
Key secondary endpoints included overall survival (OS) and pathologic complete response (pCR) at the time of cystectomy.
Additional assessments included safety, tolerability, and perioperative outcomes.
Evolution of the Study Design
The structure of KEYNOTE-905/EV-303 evolved over time to better reflect real-world clinical practice:
- 2019: Trial initiated as a two-arm study evaluating perioperative pembrolizumab versus surgery alone
- 2020: A third arm was added to include perioperative enfortumab vedotin + pembrolizumab, resulting in 1:1:1 randomization
- 2022: The pembrolizumab-alone arm was discontinued, and the study moved to a 1:1 randomization between EV + pembrolizumab and surgery alone
- Eligibility criteria were subsequently expanded to include patients who were technically cisplatin-eligible but declined cisplatin
These adaptations increased the applicability of the results to contemporary clinical practice.
Results
A total of 344 patients were enrolled in the KEYNOTE-905/EV-303 trial, with 170 assigned to the enfortumab vedotin plus pembrolizumab arm and 174 to the surgery-alone control arm. At a median follow-up of 25.6 months, the combination regimen produced significant improvements across all major clinical endpoints.
Event-Free Survival
Event-free survival was substantially improved with EV plus pembrolizumab. The median EFS was not reached in the investigational arm, compared with 15.7 months for patients who underwent surgery alone. This translated into a 60% reduction in the risk of progression, recurrence, or death (HR 0.40; p < 0.0001).
Landmark analyses reinforced the durability of benefit:
- At 12 months, EFS rates were 77.8% with EV + pembrolizumab versus 55.1% with surgery alone.
- At 24 months, rates remained high at 74.7% compared with 39.4% in the control group.
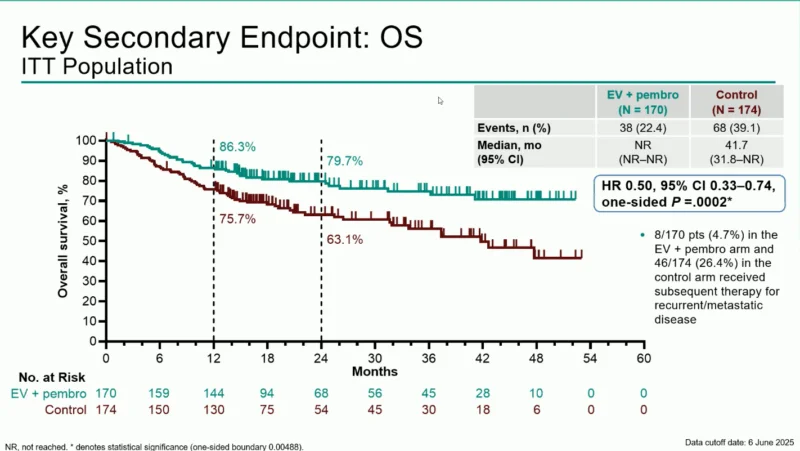
Overall Survival
Overall survival also favored the combination therapy. The median OS was not reached in the EV + pembrolizumab group, whereas it was 41.7 months in the control group (HR 0.50; p = 0.0002).
OS rates at landmark time points were:
- 12 months: 86.3% vs 75.7%
- 24 months: 79.7% vs 63.1%
These findings highlight a 50% reduction in the risk of death with the perioperative combination.
Pathologic Complete Response
The pathological response data were striking. A pathologic complete response (pCR) was achieved in 57.1% of patients treated with EV + pembrolizumab, compared with only 8.6% in the surgery-alone arm—an absolute improvement of 48.3%. This represents one of the highest pCR rates ever reported in muscle-invasive bladder cancer.
Safety
As expected with an intensified perioperative regimen, treatment-emergent adverse events were more common in the EV + pembrolizumab arm. All patients (100%) experienced at least one adverse event, compared with 64.8% in the control arm. Grade ≥3 events occurred in 71.3% versus 45.9%, respectively.
The most notable toxicities included:
- Severe immune-mediated skin reactions attributed to pembrolizumab (11.4%)
- Cutaneous toxicities typical of enfortumab vedotin (10.8%)
Despite the increased toxicity burden, adverse events were generally manageable, consistent with previously known profiles, and no new safety signals emerged.
Interpretation
KEYNOTE-905/EV-303 demonstrates that perioperative enfortumab vedotin combined with pembrolizumab delivers clinically meaningful and statistically significant improvements in event-free survival, overall survival, and pathologic response in patients who are ineligible for or decline cisplatin-based therapy.
These results establish the combination as the first perioperative systemic regimen to outperform radical cystectomy alone in this population, marking a transformative advance and defining a new standard of care for cisplatin-ineligible muscle-invasive bladder cancer.
FDA Approval Summary (November 21, 2025)
The FDA approved pembrolizumab or pembrolizumab-Qlex combined with enfortumab vedotin-ejfv as:
• Neoadjuvant therapy prior to cystectomy
• Followed by adjuvant pembrolizumab + enfortumab vedotin, then pembrolizumab alone
for adults with muscle-invasive bladder cancer who are ineligible for cisplatin.
Regulatory Highlights
- Approval based on KEYNOTE-905/EV-303
- Significant improvements in EFS and OS
- Approval evaluated under Project Orbis, enabling parallel review internationally
- Granted priority review due to high unmet need
Approved Dosing Overview
Neoadjuvant phase (9 weeks):
- Pembrolizumab 200 mg IV q3w
- Enfortumab vedotin-ejfv 1.25 mg/kg IV on days 1 and 8 q3w × 3 cycles
Adjuvant phase:
- EV for 6 cycles q3w
- Pembrolizumab for 14 cycles q3w or 7 cycles q6w
→ Followed by pembrolizumab monotherapy until completion of 42-week adjuvant plan


