Non-muscle invasive bladder cancer (NMIBC) that becomes unresponsive to Bacillus Calmette-Guérin (BCG) represents a significant therapeutic challenge. Traditionally, patients with carcinoma in situ (CIS), with or without papillary tumors, who are ineligible for or decline radical cystectomy (RC) have had very limited bladder-sparing options. However, the recent INLEXZO FDA Approval marks an important milestone in the management of these high-risk patients. INLEXZO™ (gemcitabine intravesical system, formerly TAR-200) provides a novel intravesical, sustained-release therapy that delivers continuous local exposure to gemcitabine within the bladder. Supported by robust clinical trial outcomes and regulatory validation, this approval expands the therapeutic landscape and offers a much-needed, effective alternative for patients seeking bladder preservation (Johnson & Johnson, 2025a).
Read About Bladder Cancer on OncoDaily
SunRISe-1 Clinical Trial
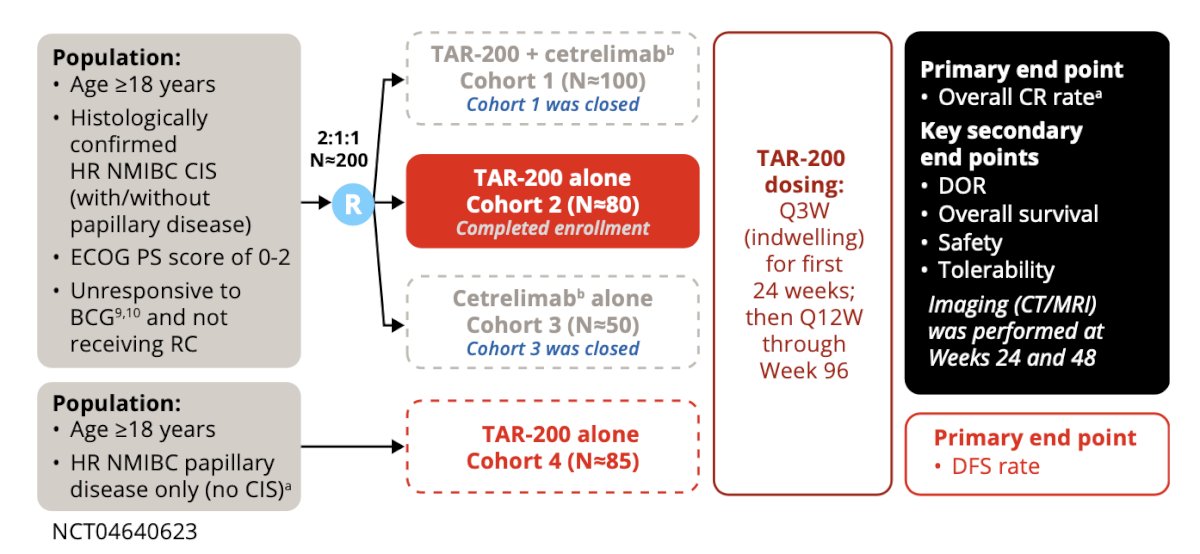
The SunRISe‑1 trial (NCT04640623) is a pivotal, open-label, randomized Phase 2b clinical study evaluating TAR-200 (now branded as INLEXZO™) with or without cetrelimab, in patients with high-risk non-muscle invasive bladder cancer (HR-NMIBC) who are BCG-unresponsive and ineligible for or decline radical cystectomy (RC).
Eligibility Overview
To participate, individuals had to be adults (18+ years) with a histologically confirmed diagnosis of persistent or recurrent high-risk NMIBC—specifically carcinoma in situ (CIS), with or without papillary disease—within 12 months of their last Bacillus Calmette-Guerin (BCG) therapy. Candidates must have received adequate BCG, defined as at least five out of six doses in the induction phase and two out of three in maintenance, or a second induction course with at least two doses. Those with T1 disease on biopsy needed to show presence of muscularis propria to rule out muscle-invasive bladder cancer (MIBC).
Participants must also have had all visible papillary disease fully resected prior to randomization, with residual CIS allowed in certain cohorts. Importantly, individuals with neuroendocrine, micropapillary, signet ring cell, plasmacytoid, or sarcomatoid histology were excluded, as were those with a history of muscle-invasive or metastatic urothelial carcinoma. Other exclusions included active hepatitis B or C, recent live virus vaccination, and prior PD-1 or PD-L1 immunotherapy.
Study Design
SunRISe‑1 follows a parallel assignment model and is non-blinded (open-label). The primary goal is to evaluate treatment efficacy and safety in four experimental cohorts, each testing different combinations of TAR-200 and cetrelimab, a PD-1 checkpoint inhibitor developed by Janssen.
Cohort 1 receives both TAR-200 and cetrelimab, targeting CIS patients with or without papillary disease.
Cohort 2 receives TAR-200 alone under the same disease criteria.
Cohort 3 administers cetrelimab alone.
Cohort 4 is specific to patients with papillary disease only, treated with TAR-200.
TAR-200 is administered intravesically via a urinary catheter every three weeks for six months, then every 12 weeks through nearly two years. Cetrelimab, where applicable, is dosed every three weeks for up to 18 months.
Primary & Secondary Outcomes
The primary endpoints vary by cohort. For Cohorts 1–3 (CIS cohorts), the focus is on overall complete response (CR) rate, defined as the absence of high-grade disease as confirmed by cystoscopy and urine cytology. For Cohort 4 (papillary only), the primary endpoint is disease-free survival (DFS)—the time until recurrence, progression, or death.
Key secondary endpoints include:
Duration of response (DoR) — time from first CR to recurrence or death.
Overall survival (OS) — time from treatment start to death from any cause.
Pharmacokinetics — measuring gemcitabine and its metabolite dFdU in urine and plasma.
Immunogenicity — incidence and concentration of anti-cetrelimab antibodies.
Patient-reported outcomes — using validated tools like EORTC QLQ-C30 and EORTC QLQ-NMIBC24 to assess quality of life.
Safety — tracking adverse events (AEs) and their severity, graded from mild (Grade 1) to death (Grade 5).
Efficacy Outcomes
According to trial results, 82% of evaluable patients achieved a complete response (CR), with a 95% confidence interval of 72%–90% (Johnson & Johnson, 2025a). Among these responders, 51% maintained CR for at least one year, highlighting the durability of response (Johnson & Johnson, 2025a). These figures represent a significant advancement for patients seeking non-surgical treatment alternatives.
Notably, 7 patients (8%) progressed to muscle-invasive disease (≥T2). Three of these had progression at cystectomy, and the median time to progression after identification of persistent or recurrent CIS or T1 disease was approximately 94 days (ClinicalTrials.gov, 2025).
Safety Profile
INLEXZO demonstrated a manageable safety profile. The most common adverse events (≥15%) included urinary frequency, urgency, dysuria, urinary tract infections, hematuria, and bladder irritation. Laboratory abnormalities were also reported, including decreased hemoglobin and lymphocytes, elevated creatinine and potassium, and increased liver enzymes (Johnson & Johnson, 2025a).
Serious adverse reactions occurred in 24% of patients. Fatal adverse events were observed in about 1.2%, including cases of cognitive disorders (Johnson & Johnson, 2025a).
Clinical Significance
This approval addresses a longstanding gap in treatment. Radical cystectomy, while effective, carries substantial morbidity and a perioperative mortality rate of 3–8%—a concern particularly for elderly or medically frail patients (Johnson & Johnson, 2025a). INLEXZO offers a viable, bladder-sparing alternative with meaningful response rates and a tolerable safety profile.
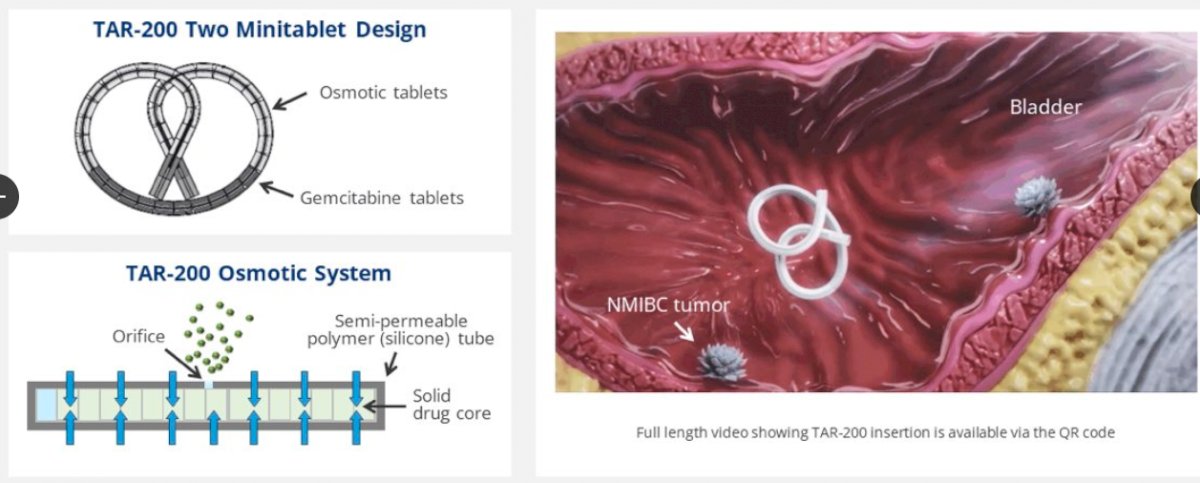
As a sustained-release intravesical gemcitabine system, INLEXZO allows for prolonged exposure to chemotherapy directly in the bladder, potentially enhancing therapeutic effects while minimizing systemic toxicity. Its minimally invasive, outpatient delivery further improves the patient experience (Johnson & Johnson, 2025a).
Conclusion
INLEXZO’s FDA approval marks a major step forward in NMIBC treatment. With high complete response rates and sustained disease control in a significant proportion of patients, it may become a preferred option for those unwilling or unable to undergo radical cystectomy. Ongoing trials will determine its role in broader NMIBC and muscle-invasive disease settings, but early data suggest INLEXZO could redefine care standards for BCG-unresponsive bladder cancer.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


