Cemiplimab achieved a landmark milestone in oncology when the U.S. Food and Drug Administration (FDA) granted approval on October 8, 2025, as the first immunotherapy for the adjuvant treatment of adults with high-risk cutaneous squamous-cell carcinoma (CSCC) following surgery and radiation. This decision was driven by the pivotal results of the Phase III C-POST trial, which demonstrated that adjuvant cemiplimab reduced the risk of recurrence or death by 68% compared with placebo.
The approval represents a turning point in the management of CSCC—a disease that, while often curable in its early stages, poses a significant threat of relapse in patients with aggressive pathological features. By extending the benefits of PD-1 blockade into the postoperative setting, cemiplimab offers a new layer of protection against recurrence and marks a crucial advancement in curative-intent skin cancer therapy.
Background
Cutaneous squamous-cell carcinoma (CSCC) is the second most common form of skin cancer, accounting for an estimated 2.4 million new cases annually worldwide. While surgery with or without adjuvant radiotherapy cures the majority of patients, a subset remains at high risk for recurrence due to aggressive pathological features.
In the C-POST trial, patients were classified as having high-risk disease based on specific nodal and nonnodal features associated with an elevated risk of recurrence after surgery and radiotherapy. High-risk nodal disease is defined as extracapsular extension with at least one lymph node measuring ≥20 mm in diameter or three or more involved nodes, regardless of extracapsular extension.
High-risk nonnodal disease included any of the following: in-transit metastases, radiologic or clinical evidence of perineural invasion of named nerves, T4 primary tumors with bone invasion, or local recurrence with at least one additional adverse feature—such as nodal stage ≥N2b, T3 lesion (>4 cm), or poorly differentiated histology in recurrent tumors ≥20 mm.These characteristics identify patients most likely to relapse despite curative surgery and adjuvant radiotherapy.
What Drug is Cemiplimab and How does it work?
Cemiplimab (Libtayo®) is a fully human monoclonal antibody developed by Regeneron Pharmaceuticals that targets the programmed cell death-1 (PD-1) receptor on T cells. It belongs to the class of immune checkpoint inhibitors, a group of cancer therapies designed to reactivate the body’s immune system against tumors.
Under normal conditions, PD-1 acts as a regulatory “brake,” preventing T cells from attacking healthy tissues. However, many cancer cells exploit this mechanism by expressing PD-L1 or PD-L2, ligands that bind to PD-1 and deactivate immune responses. Cemiplimab works by blocking the interaction between PD-1 and its ligands (PD-L1 and PD-L2), effectively releasing this brake and allowing T cells to recognize and destroy cancer cells.
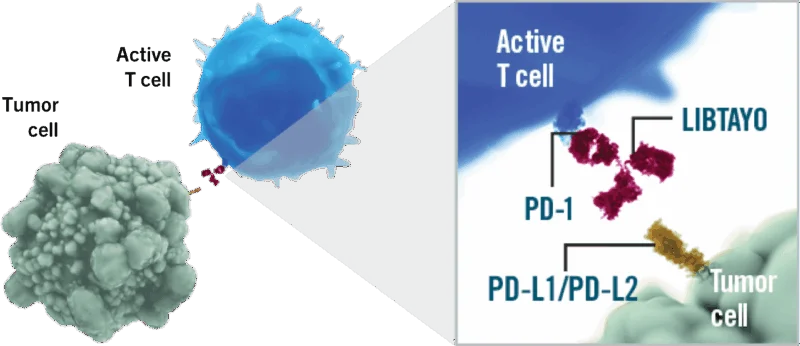
Source: LIBTAYO Official Website
Unlike traditional chemotherapy, which directly kills tumor cells, cemiplimab stimulates the patient’s immune system to mount a sustained anti-tumor response. This mechanism has shown durable clinical benefit across several cancers—including cutaneous squamous-cell carcinoma (CSCC), basal cell carcinoma (BCC), non–small cell lung cancer (NSCLC), and cervical cancer—with an expanding role in both advanced and adjuvant treatment settings.

You can read more information about Cemiplimab here.
About C-POST trial
C-POST (NCT03969004) was an international, randomized, double-blind, placebo-controlled trial enrolling 415 patients from 107 sites across 16 countries. Eligible participants had resected local or regional CSCC with high-risk nodal or non-nodal features and had completed postoperative radiotherapy within 2 to 10 weeks before randomization. Patients were assigned in a 1:1 ratio to receive either cemiplimab or placebo.
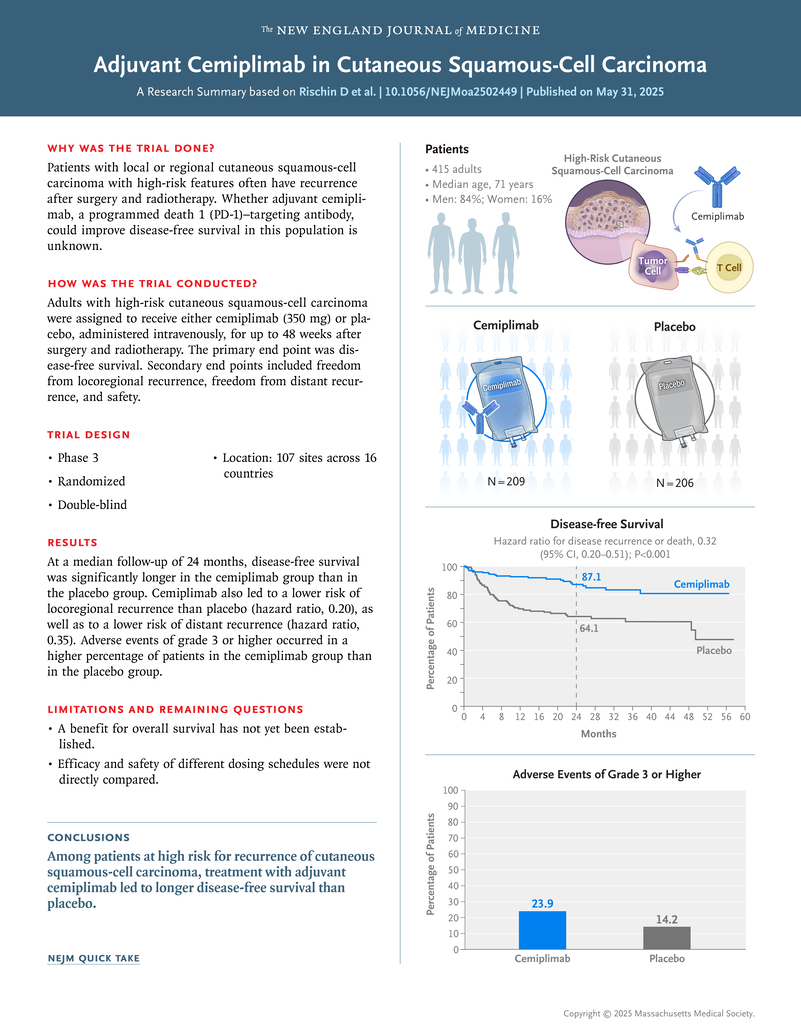
Treatment consisted of cemiplimab 350 mg intravenously every 3 weeks for 12 weeks, followed by 700 mg every 6 weeks for up to 36 additional weeks (total ≤ 48 weeks), or matching placebo. Randomization was stratified by tumor location, geographic region, high-risk category, ECOG performance status (0–1), and history of chronic lymphocytic leukemia. The primary endpoint was disease-free survival (DFS); key secondary endpoints included freedom from locoregional recurrence, freedom from distant recurrence, and safety.
Results
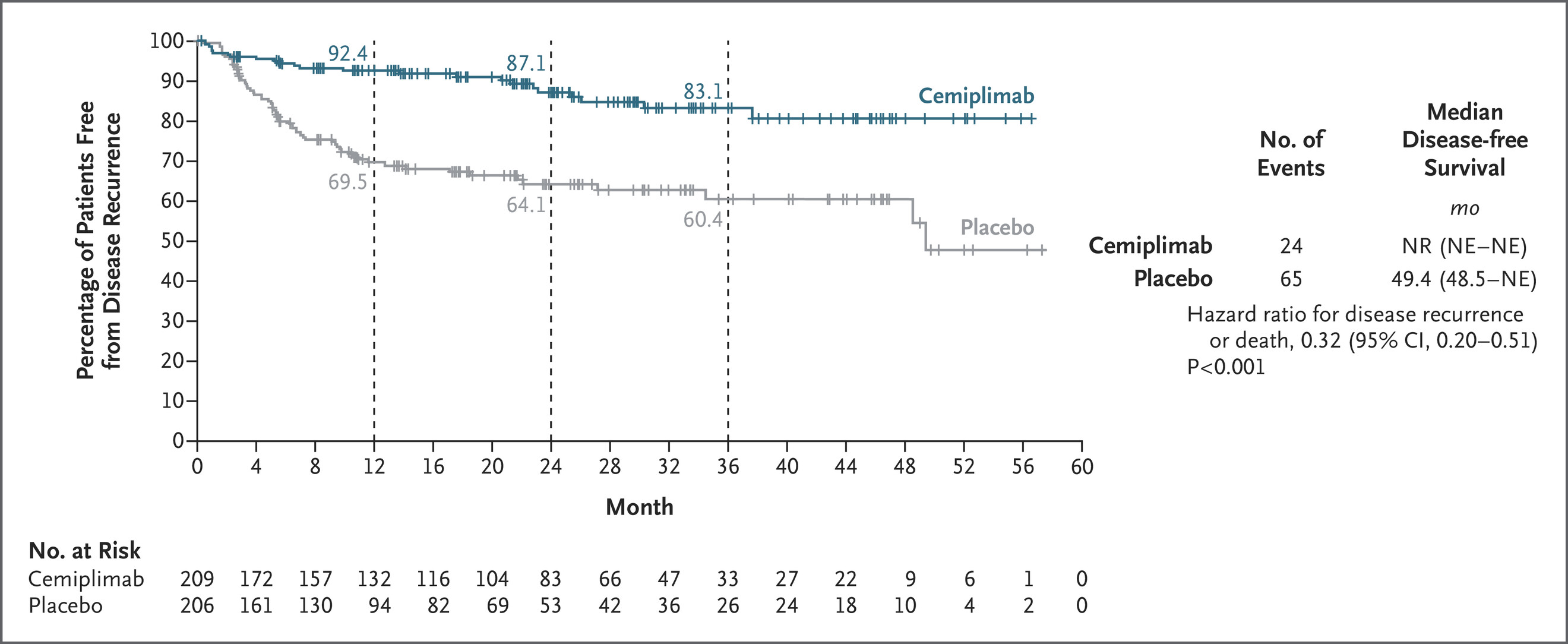
After a median follow-up of 24 months, cemiplimab significantly improved DFS compared with placebo.
- Events of recurrence or death: 24 vs 65
- Hazard ratio (HR): 0.32 [95% CI 0.20–0.51]; P < 0.001
- 24-month DFS: 87.1% (95% CI 80.3–91.6) with cemiplimab vs 64.1% (95% CI 55.9–71.1) with placebo
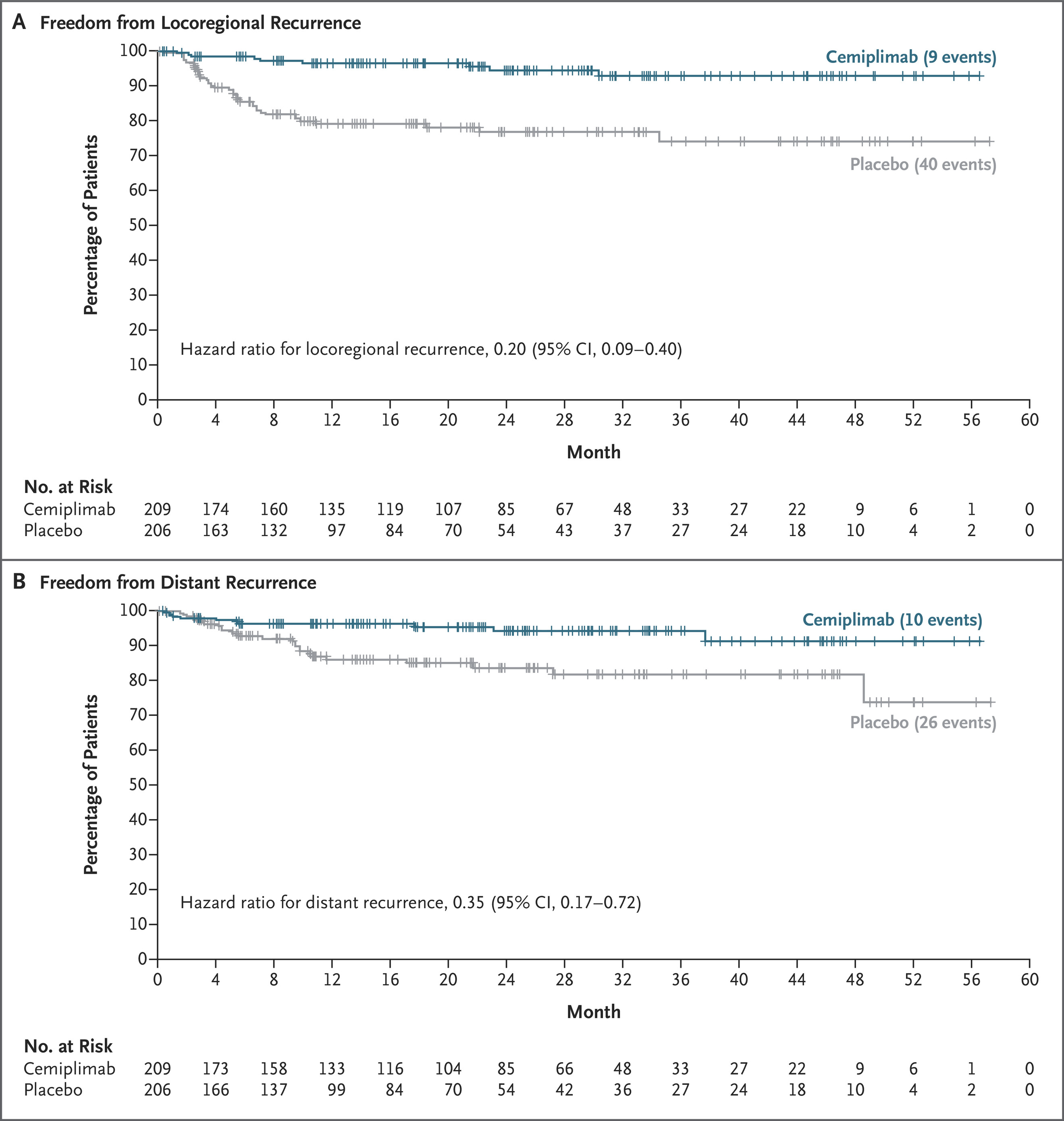
Cemiplimab also reduced both locoregional and distant recurrence:
- Locoregional recurrence: 9 vs 40 events (HR 0.20; 95% CI 0.09–0.40)
- Distant recurrence: 10 vs 26 events (HR 0.35; 95% CI 0.17–0.72)
At the time of analysis, the median DFS was not reached in the cemiplimab arm, whereas it was 49.4 months in the placebo arm. The benefit of adjuvant cemiplimab was consistent across subgroups, including patients with PD-L1 tumor proportion scores ≥ 1% and < 1%.
At 24 months, overall survival was 94.8% (95% CI 89.6–97.4) with cemiplimab and 92.3% (95% CI 86.5–95.7) with placebo (HR 0.86 [95% CI 0.39–1.90]); follow-up for survival continues.
Safety Profile
Treatment-related adverse events occurred in 91% of patients on cemiplimab and 89% on placebo.
Grade ≥3 events occurred in 23.9% vs 14.2%, and discontinuation due to toxicity in 9.8% vs 1.5% of patients, respectively.The most common adverse events were fatigue (22%), pruritus (16%), rash (16%), and diarrhea (15.6%).Immune-related adverse events occurred in 22.9% of cemiplimab-treated patients, with 7.3% being grade ≥3. One treatment-related death (myositis) was reported.
Patient-reported outcomes assessed using EORTC QLQ-C30 showed no clinically meaningful decline in quality of life between the two groups, indicating that adjuvant immunotherapy was generally well tolerated.
Regulatory Context
The FDA review of cemiplimab for adjuvant CSCC was conducted under the Real-Time Oncology Review (RTOR) program, designed to expedite access to promising cancer therapies. The submission also used the Assessment Aid, and the application received Priority Review designation.
The FDA prescribing information recommends cemiplimab 350 mg IV every 3 weeks for 12 weeks, followed by 700 mg every 6 weeks, or 350 mg every 3 weeks until recurrence, unacceptable toxicity, or completion of 48 weeks of therapy.
Clinical Significance of the Approval
The C-POST trial represents the first successful Phase III study to demonstrate a statistically and clinically significant improvement in disease-free survival with adjuvant immunotherapy in cutaneous SCC. Cemiplimab reduced the risk of recurrence or death by 68%, with particularly pronounced effects on locoregional control.
These findings are consistent with the established activity of PD-1 blockade in advanced or metastatic CSCC, where cemiplimab achieves objective responses in approximately 47% of patients and median response durations exceeding 40 months. The durable DFS advantage observed in C-POST now extends the therapeutic benefit of PD-1 inhibition to earlier stages of disease.
The safety profile mirrored that seen in prior cemiplimab studies, without new immune-related safety signals. Importantly, quality of life was preserved throughout therapy, supporting the feasibility of long-term PD-1 blockade in the postoperative setting.
Although an overall survival benefit has not yet been confirmed, ongoing follow-up will clarify whether earlier use of immunotherapy translates into improved long-term survival. The results stand in contrast to the KEYNOTE-630 trial of adjuvant pembrolizumab, which was halted for futility—emphasizing the unique benefit demonstrated by cemiplimab in this patient population.
Key Takeaway
Adjuvant cemiplimab significantly prolongs disease-free survival and reduces both locoregional and distant recurrence in patients with high-risk cutaneous squamous-cell carcinoma following surgery and radiation. With its FDA approval in October 2025, cemiplimab becomes the first and only immunotherapy approved in the adjuvant setting for CSCC, establishing a new global standard of care for patients at elevated risk of recurrence.
You can read the FDA approval article here.


