Cemiplimab is a fully human monoclonal antibody that targets the PD-1 receptor, offering a novel immunotherapeutic approach in the management of various malignancies. As part of the growing class of immune checkpoint inhibitors, it works by restoring T-cell–mediated antitumor activity, showing promise in patient populations with limited treatment options. With a distinct pharmacologic profile and expanding clinical relevance, cemiplimab has garnered attention for its potential role across multiple tumor types.
This article will explore cemiplimab’s clinical applications, common side effects, recommended dosing strategies, treatment expectations, and more.
Which Company Produced Cemiplimab?
Cemiplimab, marketed as Libtayo, was initially developed through a strategic collaboration between Regeneron Pharmaceuticals, Inc. and Sanofi. Regeneron, a U.S.-based biotechnology company known for its proprietary VelocImmune® antibody platform, discovered and engineered the drug. Sanofi, a multinational pharmaceutical company headquartered in France, partnered with Regeneron in 2015 to co-develop and co-commercialize cemiplimab as part of a broader immuno-oncology alliance. Under this partnership, Regeneron led U.S. commercialization, while Sanofi managed ex-U.S. markets.
In June 2022, Regeneron acquired exclusive global rights to cemiplimab from Sanofi in a deal valued at $900 million upfront, plus potential milestone payments and royalties. Since then, Regeneron has been solely responsible for the drug’s worldwide development, manufacturing, and commercialization.
Beyond Sanofi, Regeneron has entered several scientific collaborations to expand cemiplimab’s therapeutic potential. These include partnerships with BioNTech to explore combinations with mRNA cancer vaccines (e.g., BNT116, BNT111), ISA Pharmaceuticals for trials combining Libtayo with HPV-targeted immunotherapy (ISA101), and SillaJen to investigate synergy with the oncolytic virus Pexa-Vec in renal cancer.
In early 2024, Medison Pharma struck an exclusive agreement with Regeneron to distribute Libtayo (cemiplimab) across selected European and global markets. These collaborations reflect a broader strategy to position cemiplimab at the forefront of next-generation immuno-oncology combinations.
How Does Cemiplimab Work?
Libtayo is a fully human monoclonal antibody that targets the programmed cell death-1 (PD-1) receptor, a key immune checkpoint on T cells. Under normal conditions, PD-1 helps keep the immune system from attacking healthy tissues. However, many cancer cells exploit this pathway by expressing PD-L1 or PD-L2, ligands that bind to PD-1 and “turn off” T cells, preventing them from attacking the tumor.
Cemiplimab works by blocking the interaction between PD-1 and its ligands (PD-L1 and PD-L2). By doing so, it releases the brakes on the immune system, allowing T cells to recognize and destroy cancer cells more effectively. This immune reactivation can lead to tumor shrinkage and disease control in several types of cancers. As part of the class of immune checkpoint inhibitors, cemiplimab restores anti-tumor immune activity without directly targeting the tumor itself—making the patient’s immune system the primary weapon against the cancer.
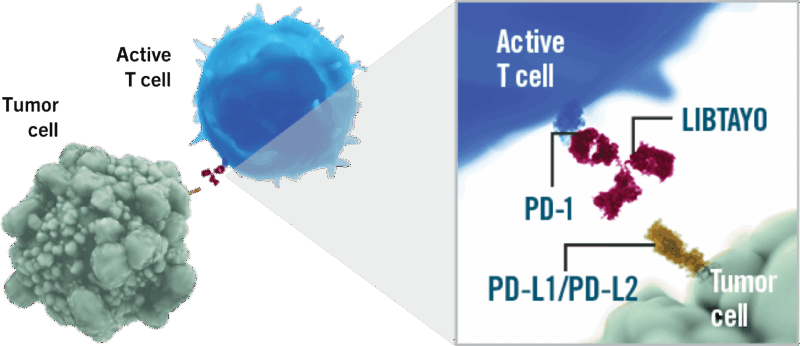
Source: LIBTAYO Official Website
What Cancers Is Cemiplimab Approved to Treat?
Cemiplimab, marketed as Libtayo, has received multiple FDA approvals for the treatment of advanced cancers. Its approvals span both skin and lung cancers, offering new therapeutic options for patients with limited alternatives.
Cemiplimab for Skin Cancer
Libtayo was first approved in September 2018 for patients with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) who are not candidates for curative surgery or radiation. This was the first time the FDA approved a treatment specifically for advanced CSCC, marking a major step forward for immunotherapy in skin cancer.
In February 2021, the FDA extended its approval to include advanced basal cell carcinoma (BCC). Cemiplimab is indicated for patients with locally advanced BCC who were previously treated with a hedgehog pathway inhibitor (HHI) or for whom such treatment is not appropriate. The same approval also included accelerated approval for treating metastatic BCC in similar patients, making cemiplimab the first immunotherapy approved for this indication.
Cemiplimab for Lung Cancer
Cemiplimab also plays a significant role in the treatment of advanced non–small cell lung cancer (NSCLC). In February 2021, it was approved as a first-line monotherapy for patients with NSCLC whose tumors express PD-L1 ≥50%, and who do not have EGFR, ALK, or ROS1 mutations. This indication is for patients with metastatic or locally advanced disease who are not eligible for surgery or definitive chemoradiation.
Later, in November 2022, the FDA approved cemiplimab in combination with platinum-based chemotherapy for the first-line treatment of advanced NSCLC, regardless of PD-L1 expression or tumor histology. This expanded indication makes cemiplimab available to a broader group of patients without actionable mutations and who are unsuitable for curative treatment approaches.
What research is behind the approval?
The FDA approvals of Libtayo were based on pivotal clinical trials demonstrating durable tumor responses, significant survival benefits, and a manageable safety profile in patients with advanced cutaneous squamous cell carcinoma, basal cell carcinoma, and non-small cell lung cancer.”
Cemiplimab for advanced cutaneous squamous cell carcinoma
Approval of Libtayo for advanced cutaneous squamous cell carcinoma (CSCC) was supported by two clinical trials that enrolled patients with metastatic or locally advanced disease who were not candidates for curative treatment. Among 108 patients, the overall response rate (ORR) was 47%, with durable responses seen in over 60% of responders lasting six months or longer. Clinical benefit was observed across both metastatic and locally advanced subtypes, with visible tumor shrinkage noted in many cases.
The safety profile was consistent with other PD-1 inhibitors. Common side effects included fatigue, rash, and diarrhea, while serious immune-mediated reactions such as pneumonitis, colitis, hepatitis, and endocrinopathies were also reported. Cemiplimab is given as a 350 mg IV infusion every three weeks.
Read more about Immunotherapy for Skin Cancer: Types, Success Rate, Side Effects on OncoDaily.
Cemiplimab in Locally Advanced Basal Cell Carcinoma
In 2021, results published in The Lancet Oncology highlighted the clinical efficacy of cemiplimab in patients with locally advanced basal cell carcinoma (laBCC) who had previously received and discontinued hedgehog inhibitor (HHI) therapy due to progression or intolerance.
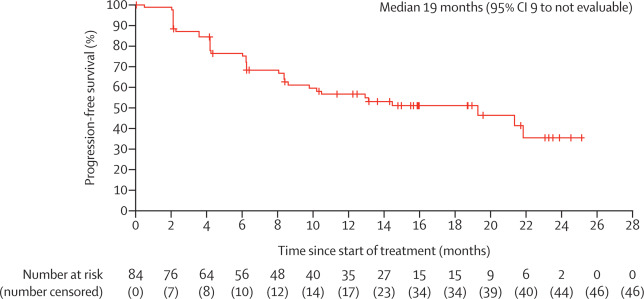
This open-label, multicenter, single-arm phase 2 trial (NCT03132636) enrolled 84 patients across North America and Europe. Patients received cemiplimab 350 mg IV every 3 weeks for up to 93 weeks. At a median follow-up of 15 months, 31% of participants achieved an objective response, with 6% complete responses and 25% partial responses. Notably, disease control was observed in 80% of patients, and nearly half maintained response for over a year.
Cemiplimab showed a manageable safety profile, with Grade 3–4 treatment-related adverse events in 48% of patients, most commonly hypertension and colitis. Importantly, no treatment-related deaths occurred. These findings support Libtayo as a meaningful option for laBCC patients after HHI failure, offering durable responses and manageable toxicity. The study reinforces its therapeutic role in non-melanoma skin cancers beyond cutaneous squamous cell carcinoma.
Cebiplimab for Non Small Cell Lung Cancer
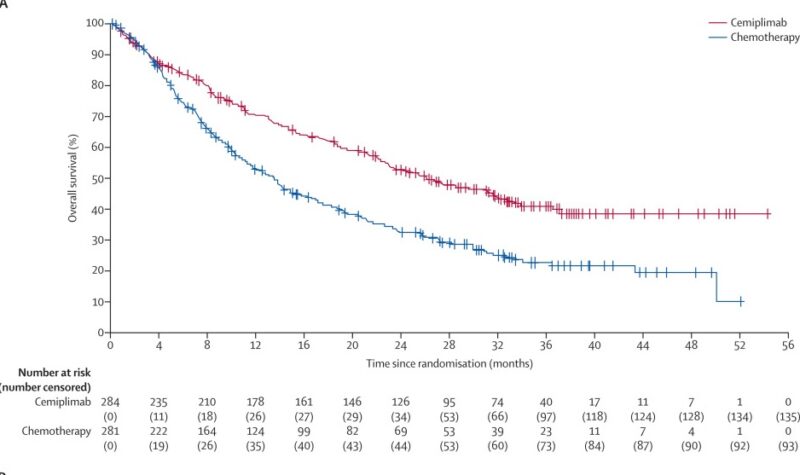
In 2023, The Lancet Oncology published the 35-month follow-up results of the EMPOWER-Lung 1 trial, which evaluated cemiplimab in advanced non-small-cell lung cancer (NSCLC) patients with PD-L1 expression of 50% or higher. The study enrolled 712 patients who were randomized to receive either cemiplimab or chemotherapy. Libtayo significantly improved median overall survival, extending it to 26.1 months compared to 13.3 months with chemotherapy (hazard ratio [HR] 0.57). Progression-free survival was also longer with cemiplimab (8.1 vs. 5.3 months; HR 0.51).
For patients who progressed on cemiplimab, adding chemotherapy while continuing cemiplimab resulted in a median progression-free survival of 6.6 months and overall survival of 15.1 months. Grade 3–4 treatment-related adverse events such as anemia and neutropenia were less common in the cemiplimab group. The safety profile remained consistent over time with no new safety concerns. These findings confirm cemiplimab’s durable survival benefit as first-line monotherapy and suggest that combining chemotherapy at progression may offer an effective second-line option.
Cemiplimab Combinations and Treatment Outcomes
The randomized, double-blind, phase 3 EMPOWER-Lung 3 trial, published in Nature Medicine and indexed in PubMed (PMID: 36008722), evaluated cemiplimab combined with platinum-doublet chemotherapy versus chemotherapy alone as first-line treatment for advanced non-small cell lung cancer (aNSCLC), regardless of PD-L1 expression or histology. The study enrolled 466 patients without EGFR, ALK, or ROS1 alterations, randomizing them 2:1 to cemiplimab plus chemotherapy or placebo plus chemotherapy.
The trial was stopped early due to a clear survival benefit with cemiplimab. Median overall survival (OS) reached 21.9 months in the cemiplimab arm versus 13.0 months in the control group (hazard ratio [HR] 0.71, P = 0.014). Progression-free survival (PFS) also improved significantly with cemiplimab plus chemotherapy, showing a median of 8.2 months compared to 5.0 months with chemotherapy alone (HR 0.56, P < 0.0001). Objective response rate (ORR) was higher in the cemiplimab group at 43.3%, with a median duration of response (DOR) of 15.6 months versus 22.7% and 7.3 months in controls.
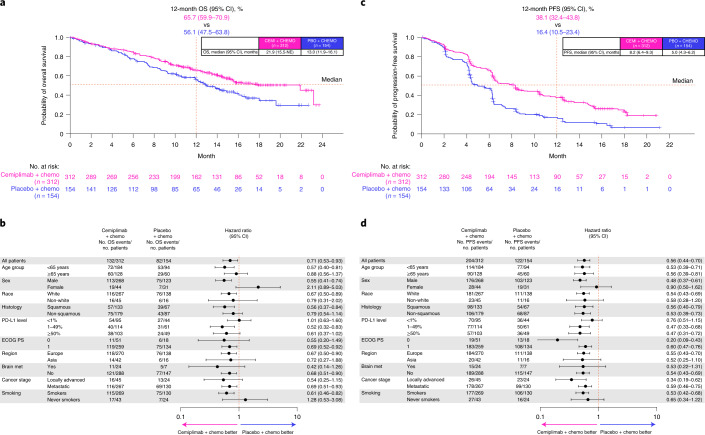
Benefits in OS, PFS, and ORR were consistent across subgroups including squamous and non-squamous histologies, with the exception of smaller subsets like women and never-smokers. Patient-reported outcomes showed a trend toward improved quality of life with cemiplimab plus chemotherapy. Grade 3 or higher adverse events were more frequent in the cemiplimab arm (43.6% vs. 31.4%), but safety was manageable. These findings confirm that cemiplimab combined with platinum-based chemotherapy significantly improves survival outcomes compared to chemotherapy alone in aNSCLC, supporting its use as a first-line option regardless of PD-L1 status.
Cemiplimab side effects and its management
Immune checkpoint inhibitors like Libtayo can provoke a broad spectrum of adverse events due to immune activation. While many patients tolerate treatment well, immune-related toxicities may affect multiple organ systems and range from mild to severe. Early identification and appropriate management are essential to minimize morbidity.
Common Side Effects
The most commonly reported adverse effects with Libtayo include fatigue, rash, diarrhea, nausea, musculoskeletal pain, and pruritus. These events generally occur in 10–30% of patients and are mostly low to moderate in severity. Fatigue is often persistent but manageable with supportive care. Dermatologic toxicities range from mild rash to pruritus and may require topical corticosteroids or antihistamines. Gastrointestinal symptoms such as diarrhea are usually mild but should be closely monitored to differentiate from immune-mediated colitis. Laboratory abnormalities, including anemia and elevated liver enzymes, have also been observed and typically respond well to treatment modifications.
Less Common but Clinically Significant Side Effects
While less frequent, immune-mediated toxicities involving vital organs demand heightened vigilance. Pneumonitis, presenting with cough and dyspnea, occurs in approximately 1–5% of patients and can be life-threatening if unrecognized. Hepatitis and colitis similarly require prompt identification and intervention to prevent severe complications. Endocrinopathies—hypothyroidism, hyperthyroidism, adrenal insufficiency, and diabetes mellitus—are notable for their potential permanence, often necessitating lifelong hormone replacement therapy.
Rare adverse events such as myocarditis, nephritis, hypophysitis, and severe cutaneous reactions including Stevens-Johnson syndrome or toxic epidermal necrolysis have been reported and warrant immediate drug discontinuation and specialist management. Infusion-related reactions are uncommon but may require slowing or halting the infusion.
Management of Adverse Events
Management typically follows established irAE guidelines. For mild (Grade 1) events, treatment may be continued with close monitoring. Moderate (Grade 2) events often require temporary discontinuation and corticosteroids. Severe (Grade 3 or 4) toxicities usually necessitate permanent discontinuation and high-dose corticosteroids, with additional immunosuppressants considered in steroid-refractory cases. Endocrine irAEs are typically irreversible and managed with long-term hormone replacement. Prompt evaluation and multidisciplinary coordination are crucial in managing complex cases.
What is the Recommended Dosage of Cemiplimab?
Libtayo is typically dosed at 350 mg every three weeks across most approved indications, including cutaneous squamous cell carcinoma (CSCC), basal cell carcinoma (BCC), and non–small cell lung cancer (NSCLC). No weight-based dosing is used, simplifying administration. Treatment continues until disease progression or unacceptable toxicity, with no established maximum number of cycles. In combination regimens (e.g., with chemotherapy in NSCLC), the timing aligns with chemo cycles. Dose modifications are not routinely recommended, but treatment may be delayed or discontinued for immune-related adverse events.
How is Cemiplimab administered?
Cemiplimab is given as an intravenous infusion, meaning it’s delivered directly into the bloodstream through a vein. Before it’s administered, the solution from the vial is checked to ensure it’s clear to slightly opalescent and free of unusual particles—anything cloudy or discolored must be discarded. The required dose is drawn up and diluted in either normal saline (0.9% sodium chloride) or 5% dextrose in water (D5W), then gently inverted to mix—never shaken. Once prepared, the solution is infused over 30 minutes using a line fitted with a sterile filter.
If the diluted solution is not used immediately, it can be stored in the refrigerator for up to 24 hours or kept at room temperature (below 25°C) for up to 8 hours, but it must reach room temperature before administration. Both the vial and the diluted infusion must be protected from freezing and from excessive heat, and they should be shielded from light. This careful preparation and handling process ensures the safety and effectiveness of cemiplimab therapy.
Cemiplimab effectiveness over time
The final results of the phase III EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9 trial, published in Annals of Oncology (ESMO), confirmed that cemiplimab significantly improved overall survival in patients with recurrent cervical cancer compared to chemotherapy. With a median follow-up of over 47 months, median OS was 11.7 months with cemiplimab versus 8.5 months with chemotherapy (HR 0.67; p < 0.00001). The benefit was observed regardless of PD-L1 status. The safety profile remained consistent, supporting cemiplimab as a second-line option in this setting.
You can read about Immunotherapy for Cervical Cancer: Types, Success Rate, Side Effects on OncoDaily.
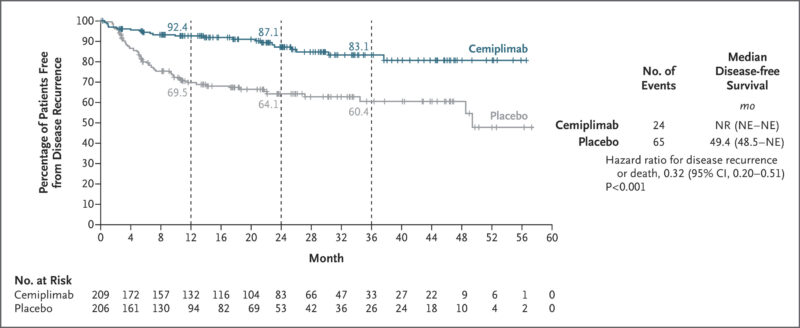
In the phase 3 C-POST trial, published in The New England Journal of Medicine in 2025, adjuvant Libtayo significantly improved disease-free survival in patients with high-risk cutaneous squamous cell carcinoma (CSCC) who had undergone surgery and radiotherapy. At 24 months, disease-free survival was 87.1% with cemiplimab vs. 64.1% with placebo (HR 0.27). The treatment also reduced locoregional recurrence (HR 0.28) and distant metastasis (HR 0.24). Grade ≥3 adverse events occurred in 22.5% of cemiplimab-treated patients vs. 11.1% with placebo.
Ongoing trials with Cemiplimab
The ARCH trial (NCT06931717) is a multicentre, phase III study evaluating adjuvant Libtayo in patients with resected stage II–IIIA non-small cell lung cancer (NSCLC) who have not received prior chemotherapy. Participants receive Libtayo intravenously on a scheduled regimen, with treatment continuing until relapse or toxicity. The trial’s main goal is to assess disease-free survival in patients whose tumors express PD-L1 ≥1%, comparing outcomes with a non-treatment observation group. The study is sponsored by ETOP IBCSG Partners Foundation.
The Phoenix Trial (NCT05961709), led by MD Anderson Cancer Center, is a phase II study evaluating neoadjuvant Libtayo as a non-surgical treatment for localized dMMR colon cancer. The trial explores whether a single dose of cemiplimab can enable organ preservation by achieving clinical response without surgery. Key goals include assessing complete endoscopic response, radiographic and pathological outcomes, survival rates, safety, and molecular predictors of response. The trial also examines immune and genomic markers to better understand treatment efficacy and patient selection.
FAQ
What type of drug is Cemiplimab?
Cemiplimab is a fully human monoclonal antibody and an immune checkpoint inhibitor. It blocks the PD-1 receptor on T cells, helping the immune system recognize and attack cancer cells.
Which cancers is Libtayo currently approved to treat?
Libtayo is FDA-approved for advanced cutaneous squamous cell carcinoma (CSCC), basal cell carcinoma (BCC), non–small cell lung cancer (NSCLC), and recurrent cervical cancer.
What is the recommended dosage of Libtayo?
The standard dosage is 350 mg every three weeks, regardless of body weight, until disease progression or unacceptable side effects occur.
What are the most common side effects of Cemiplimab?
Patients often report fatigue, rash, diarrhea, nausea, and joint or muscle pain. Most side effects are manageable, but serious immune-related reactions can occur.
How does Libtayo differ from chemotherapy?
Unlike chemotherapy, which directly kills cancer cells, Cemiplimab stimulates the immune system to attack cancer. This can mean fewer traditional chemo side effects but a higher risk of immune-related toxicities.
How effective is Cemiplimab in treating lung cancer?
Clinical trials have shown that Libtayo improves overall survival compared to chemotherapy in advanced NSCLC, both as a single agent (for PD-L1–positive tumors) and in combination with chemotherapy.
What is the half-life of Cemiplimab?
Libtayo has a half-life of about 19 days.