Immunotherapy is emerging as a groundbreaking treatment for cervical cancer, particularly in advanced and metastatic stages, where traditional therapies like chemotherapy and radiation often have limited success. By harnessing the body’s immune system, immunotherapy offers new hope for patients with recurrent or treatment-resistant cervical cancer. This article will explore the types of immunotherapy used in cervical cancer treatment, success rates, potential side effects, and the financial aspects of these therapies.
What Is Immunotherapy for Cervical Cancer?
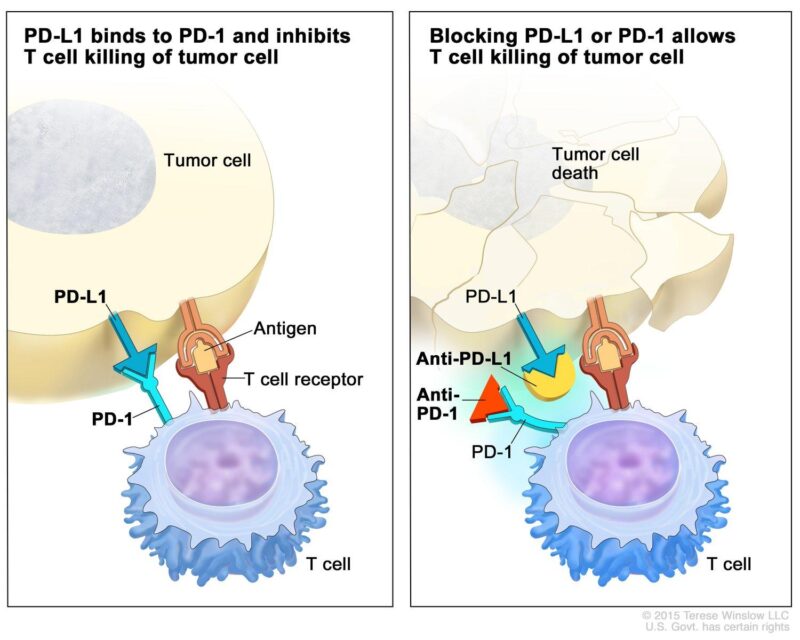
Immunotherapy is a form of cancer treatment that enhances the body’s immune system to identify and attack cancer cells more effectively. In the context of cervical cancer, immunotherapy has gained attention, particularly with the use of immune checkpoint inhibitors like pembrolizumab (Keytruda).
Pembrolizumab is a monoclonal antibody that targets the programmed death-1 (PD-1) protein found on T cells, a type of immune cell. Under normal circumstances, PD-1 interacts with its ligand, PD-L1, which can be expressed on tumor cells, leading to the suppression of the immune response against these cells. By blocking the PD-1/PD-L1 interaction, pembrolizumab reactivates T cells, enabling them to recognize and destroy cancer cells more effectively.
What Are the Types of Immunotherapy for Cervical Cancer?
Several types of immunotherapies are being used in the treatment of cervical cancer, particularly in advanced and recurrent cases. These include immune checkpoint inhibitors, such as pembrolizumab (Keytruda), which block PD-1/PD-L1 pathways to enhance the immune response against cancer cells. Another promising approach is antibody-drug conjugates (ADCs), which combine a monoclonal antibody with a chemotherapy drug to deliver targeted cancer cell destruction.
Checkpoint Inhibitors
Pembrolizumab (Keytruda), a PD-1 inhibitor, has become a pivotal treatment for advanced cervical cancer, particularly in tumors expressing PD-L1. In June 2018, the U.S. Food and Drug Administration (FDA) granted accelerated approval for pembrolizumab to treat patients with recurrent or metastatic cervical cancer exhibiting PD-L1 expression (Combined Positive Score [CPS] ≥1), following disease progression after chemotherapy.
This approval was based on findings from the KEYNOTE-158 trial, a multicenter, non-randomized study involving 98 patients with advanced cervical cancer. Among the 77 patients whose tumors expressed PD-L1 (CPS ≥1), the objective response rate (ORR) was 14.3%, with 2.6% achieving complete responses and 11.7% partial responses. Notably, 91% of responders maintained their response for six months or longer.
In October 2021, the FDA expanded pembrolizumab’s approval, allowing its use in combination with chemotherapy, with or without bevacizumab, for patients with persistent, recurrent, or metastatic cervical cancer, irrespective of PD-L1 status. This decision was informed by the KEYNOTE-826 trial, which demonstrated significant improvements in progression-free and overall survival with the addition of pembrolizumab to standard chemotherapy regimens.
Antibody-Drug Conjugates (ADCs)
Tisotumab vedotin (Tivdak) is an innovative antibody-drug conjugate (ADC) designed to target and deliver cytotoxic agents directly to cancer cells, thereby minimizing damage to healthy tissues. This targeted approach has shown promise in treating advanced or recurrent cervical cancer. Tisotumab vedotin comprises a monoclonal antibody that specifically binds to tissue factor (TF), a protein commonly overexpressed on the surface of cervical cancer cells. Upon binding to TF, the ADC is internalized into the cancer cell, where it releases monomethyl auristatin E (MMAE), a potent cytotoxic agent. MMAE disrupts the microtubule network within the cell, leading to cell cycle arrest and apoptosis. This targeted delivery system enhances the efficacy of the cytotoxic agent while reducing systemic toxicity.
The efficacy of tisotumab vedotin in treating recurrent or metastatic cervical cancer was evaluated in the innovaTV 204 trial, a multicenter, open-label, single-arm, phase II study. In this trial, 101 patients who had previously received one or two systemic therapies, including at least one platinum-based regimen, were administered tisotumab vedotin at a dose of 2.0 mg/kg every three weeks until disease progression or unacceptable toxicity occurred. The study reported an objective response rate (ORR) of 24% (95% CI, 15.9–33.3), with a median duration of response of 8.3 months. These findings indicate that tisotumab vedotin has notable antitumor activity in this patient population.
Building on these results, the innovaTV 301 trial, a global, randomized, open-label, phase III study, compared tisotumab vedotin to investigator’s choice of chemotherapy in patients with recurrent or metastatic cervical cancer who had received one or two prior systemic regimens. The trial demonstrated that tisotumab vedotin resulted in a statistically significant 30% reduction in the risk of death compared to chemotherapy, highlighting its potential as an effective treatment option in this setting. Based on the clinical efficacy demonstrated in these trials, the U.S. Food and Drug Administration (FDA) granted accelerated approval to tisotumab vedotin in September 2021 for the treatment of adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy. This approval provides a new therapeutic option for patients with limited alternatives.
What Are the Side Effects of Immunotherapy for Cervical Cancer?
Immunotherapy for cervical cancer can result in a range of side effects that vary in severity. While some side effects are common and generally manageable, others can be serious and require close monitoring.

Common Side Effects
Pembrolizumab (Keytruda), a PD-1 inhibitor, has been associated with several common side effects that can significantly impact patients’ daily lives. According to a study published in the Journal of Clinical Oncology by Dr. Julie R. Brahmer et al., fatigue was reported in 40% of patients undergoing pembrolizumab treatment. This persistent tiredness can interfere with routine activities, reducing overall quality of life.
Diarrhea is another frequent side effect, affecting 27% of patients, as noted in the same study. This condition can lead to dehydration and electrolyte imbalances, necessitating dietary adjustments and, in some cases, medication to manage symptoms. Additionally, rash occurred in 30% of patients, according to the study by Dr. Brahmer and colleagues. These skin reactions, ranging from mild redness to severe dermatitis, can cause discomfort and may require topical treatments or dose modifications.
Severe Side Effects
Immune-related adverse events (irAEs), such as pneumonitis and colitis, are significant concerns for patients undergoing pembrolizumab (Keytruda) therapy.
- Immune-Related Pneumonitis: According to a study published in Frontiers in Immunology by Zhang et al., the incidence of checkpoint inhibitor-related pneumonitis (CIP) in patients treated with pembrolizumab varies, with rates reported up to 14.5%. The median time to onset of CIP was approximately 52.5 days after initiating therapy. Notably, among patients who developed CIP, 27% succumbed to the condition, highlighting its potential severity.
- Immune-Related Colitis: In a study published in ESMO Open by Alexander et al., the incidence of immune checkpoint inhibitor-induced colitis among patients receiving pembrolizumab was reported to be 3.5%. This condition often presents with symptoms ranging from frequent loose stools to more severe manifestations like abdominal pain and hematochezia.
Immunotherapy for Advanced Cervical Cancer
Recent advancements in immunotherapy have significantly improved outcomes for patients with metastatic or recurrent cervical cancer, particularly through combination treatments with chemotherapy.
- Pembrolizumab Plus Chemotherapy: The phase III KEYNOTE-826 study, led by Dr. Nicoletta Colombo and published in the New England Journal of Medicine, evaluated the efficacy of adding pembrolizumab to standard chemotherapy in patients with persistent, recurrent, or metastatic cervical cancer. The study demonstrated that the addition of pembrolizumab resulted in a 33% reduction in the risk of death compared to chemotherapy alone. Furthermore, the two-year overall survival rate was 53.0% in the pembrolizumab group versus 41.7% in the placebo group.
- Atezolizumab Plus Chemotherapy and Bevacizumab: Another significant development is the phase III BEATcc trial, led by Dr. Ana Oaknin and published in The Lancet, which investigated the addition of atezolizumab, a PD-L1 inhibitor, to standard chemotherapy and bevacizumab in untreated metastatic or recurrent cervical cancer. The study reported a median overall survival of 32.1 months in the atezolizumab group compared to 22.8 months in the control group, indicating a substantial improvement. Additionally, the two-year survival rate was 60% with atezolizumab versus less than 40% with standard therapy.
Combination Therapies
Combining immunotherapy with chemotherapy or radiation therapy has shown promising improvements in outcomes for patients with advanced cervical cancer. According to a study published this year by Dr. Domenica Lorusso and colleagues, the addition of pembrolizumab to concurrent chemoradiotherapy resulted in a significant overall survival benefit for patients with newly diagnosed, high-risk, locally advanced cervical cancer. In another study, Dr. Bradley J. Monk et al. reported that the objective response rate was significantly higher, being 94% in the group that received immunotherapy, compared to 32% in the group that did not.
Ongoing clinical trials continue to explore various combinations to enhance treatment efficacy. For instance, the IMMUNOCERV phase II trial is evaluating the combination of the immunotherapy agent PDS0101 with chemoradiation. Preliminary results have shown an 87.5% complete response rate at three months, with a 1-year disease-free survival rate of 85.7% and overall survival of 100%.
How Is Immunotherapy Administered?
Immunotherapy for cervical cancer is typically administered through intravenous (IV) infusions, allowing the medication to enter the bloodstream and activate the immune system against cancer cells. The treatment is usually given in an outpatient setting, where patients receive their infusion while seated in a clinic or hospital. The duration and frequency of immunotherapy depend on the specific drug and treatment plan. For example, pembrolizumab (Keytruda) is often given every three to six weeks, with each infusion lasting 30 to 90 minutes. Patients may continue treatment for up to two years, depending on how well they respond.
During sessions, medical staff monitor patients for any immediate side effects, such as fever, chills, or mild allergic reactions. Over time, patients may experience fatigue, nausea, or skin changes, but these are manageable with supportive care. Regular imaging scans and blood tests help doctors track the effectiveness of the treatment and make adjustments if needed. Understanding the process helps patients prepare for their immunotherapy journey with confidence.
What Should Patients Expect During Treatment?
Embarking on immunotherapy for cervical cancer involves a structured journey, beginning with an initial consultation and extending through treatment and follow-up care. The process commences with a comprehensive discussion between the patient and oncologist to assess the cancer’s stage and determine the suitability of immunotherapy. Once deemed appropriate, a personalized treatment plan is developed. This plan outlines the specific immunotherapeutic agents to be used, such as pembrolizumab, and the schedule for administration.
Throughout treatment, patients may experience side effects like fatigue, diarrhea, or skin rashes. Proactive management strategies, including medications and lifestyle adjustments, are implemented to mitigate these effects. For instance, patients are advised to maintain open communication with their healthcare team to address any new or worsening symptoms promptly. After completing the immunotherapy regimen, patients enter a follow-up phase involving periodic check-ups to detect any signs of recurrence and manage long-term side effects. This ongoing care is crucial for maintaining health and promptly addressing any emerging concerns.
How Long Does It Take to See Results from Immunotherapy?
The timeline for seeing improvements with immunotherapy varies, but most patients begin to notice changes within a few months of starting treatment. Some may experience an early response, while others require more time as their immune system gradually mounts an attack against the cancer.
Factors like the stage of cervical cancer, the specific type of immunotherapy, and individual immune response all influence how quickly results appear. For example, patients receiving checkpoint inhibitors like pembrolizumab may see tumor shrinkage or stabilization after three to six months, while those undergoing combination therapy with chemotherapy might experience faster responses.
Regular imaging scans and blood tests help doctors track progress, ensuring that the treatment is working effectively. While waiting for results can be challenging, persistence and close monitoring allow for adjustments if needed, improving long-term outcomes.
What Are the Costs of Immunotherapy for Cervical Cancer?
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is immunotherapy for cervical cancer?
Immunotherapy is a treatment that boosts the body’s immune system to recognize and attack cervical cancer cells. It is mainly used for advanced or recurrent cases.
How does immunotherapy work against cervical cancer?
Immunotherapy helps activate the immune system to fight cancer by blocking proteins that cancer cells use to hide from immune cells, allowing the body to attack the tumor.
What types of immunotherapy are used for cervical cancer?
The main types include checkpoint inhibitors (like pembrolizumab), monoclonal antibodies, cancer vaccines, and T-cell therapy. Some treatments are still in clinical trials.
Who is eligible for immunotherapy for cervical cancer?
Patients with advanced or recurrent cervical cancer, especially those with PD-L1-positive tumors, are more likely to benefit from immunotherapy. A doctor will determine eligibility based on biomarker testing.
What is the success rate of immunotherapy for cervical cancer?
Success rates vary, but studies show that checkpoint inhibitors like pembrolizumab improve survival in some patients with PD-L1-positive tumors. More research is ongoing.
What are the side effects of immunotherapy?
Common side effects include fatigue, nausea, skin rashes, and flu-like symptoms. More serious effects can involve immune-related inflammation in organs like the lungs, liver, or intestines.
Is immunotherapy used alone or with other treatments?
It is often combined with chemotherapy or other targeted therapies for better results, especially in cases where the cancer is advanced or resistant to other treatments.
How long does immunotherapy treatment last?
The duration varies, but most patients receive treatment every few weeks for several months or years, depending on how well their cancer responds.
How much does immunotherapy for cervical cancer cost?
Costs depend on the specific drug, location, and insurance coverage. Checkpoint inhibitors like pembrolizumab can cost thousands per dose, but financial assistance programs may help.
Are there clinical trials for new immunotherapy treatments?
Yes, multiple clinical trials are testing new immunotherapy drugs and combinations for cervical cancer. Patients can check with their oncologist or visit clinical trial databases for options.