Tremelimumab is an immunotherapy drug designed to help the body’s immune system recognize and attack cancer more effectively. It belongs to a class of treatments known as immune checkpoint inhibitors, which work by releasing the natural brakes on immune cells, allowing them to mount a stronger and more sustained anti-tumor response. Rather than directly targeting cancer cells like chemotherapy, tremelimumab empowers the immune system itself to do the fighting.
Tremelimumab has become especially important in combination immunotherapy strategies, where it is used alongside other immune-based treatments to enhance clinical benefit. This approach has shown meaningful impact in difficult-to-treat cancers, offering the potential for longer-lasting control compared with traditional therapies alone.
This article will walk you through how tremelimumab works, which cancers it is used to treat, its recommended dosage, possible side effects and their management, what patients can expect during treatment, and how ongoing research continues to shape its role in modern cancer care.
Which company produced Tremelimumab?
Tremelimumab, marketed as Imjudo, is developed and produced by AstraZeneca as part of its immuno-oncology portfolio. It is a monoclonal antibody targeting CTLA-4, a key immune checkpoint that normally limits T-cell activation. By blocking this pathway, tremelimumab helps strengthen the body’s immune response against cancer.

You can also read Durvalumab (Imfinzi): Uses in Cancer, Side effects, Dosage, Expectations and More on OncoDaily.
How does Tremelimumab Work?
Tremelimumab works by targeting a key immune checkpoint called CTLA-4 (cytotoxic T-lymphocyte–associated protein 4). CTLA-4 is a natural “brake” on the immune system that helps prevent T cells from becoming overactive. While this is essential for protecting healthy tissues, cancers can exploit this pathway to hide from immune attack.
By binding to CTLA-4, tremelimumab blocks this inhibitory signal and allows T cells to become fully activated. Once activated, these immune cells are better able to recognize cancer cells as abnormal and mount a stronger anti-tumor response. This effect occurs primarily during the early phase of immune activation in the lymph nodes, where T cells are first trained to recognize tumor antigens.
In combination strategies such as the STRIDE regimen, Imjudo serves as an immune “primer.” It rapidly expands and activates tumor-specific T cells with a single dose, while the partner immunotherapy (targeting the PD-1/PD-L1 pathway) helps sustain and maintain this immune response over time within the tumor microenvironment. This dual checkpoint blockade approach aims to deliver deeper and more durable anti-cancer immune activity than either mechanism alone.
What Cancers Are Treated with Tremelimumab?
Tremelimumab (Imjudo) is currently used in combination immunotherapy regimens for two major cancer types, based on key regulatory milestones reached in 2022.
- On October 24, 2022, tremelimumab in combination with durvalumab was authorized by the U.S. Food and Drug Administration for the treatment of unresectable hepatocellular carcinoma. In this setting, tremelimumab is administered as a single priming dose alongside continued durvalumab therapy, forming the STRIDE regimen. This marked the first approval of a CTLA-4–based immunotherapy combination in advanced liver cancer and established tremelimumab as a core component of dual checkpoint blockade in this disease.
- On November 11, 2022, the role of tremelimumab expanded into thoracic oncology. The combination of Imjudo and Imfinzi with platinum-based chemotherapy was authorized for patients with metastatic non-small cell lung cancer. In this setting, tremelimumab contributes to deeper immune activation when added to PD-L1 blockade and chemotherapy, reinforcing its position within intensive first-line immunotherapy strategies.
Together, these two approvals define the current clinical use of tremelimumab across advanced liver and lung cancers, while additional tumor types continue to be explored in ongoing trials.
Tremelimumab for NSCLC
On November 11, 2022, the U.S. Food and Drug Administration authorized durvalumab (Imfinzi) plus tremelimumab (Imjudo) in combination with platinum-based chemotherapy for adults with stage IV metastatic non-small cell lung cancer (NSCLC).
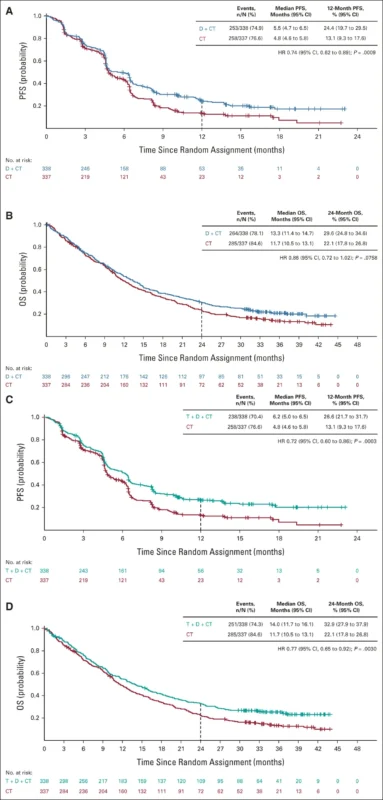
The approval was based on the POSEIDON Phase III trial, published in the Journal of Clinical Oncology in November 2022, which enrolled more than 1,000 patients with metastatic NSCLC across all PD-L1 levels and histologies. Adding a limited course of tremelimumab to durvalumab and chemotherapy reduced the risk of death by 23% and the risk of disease progression or death by 28% compared with chemotherapy alone. At two years, 33% of patients were alive with the immunotherapy-based regimen versus 22% with chemotherapy.
Longer follow-up presented at the ESMO Congress 2022 confirmed that the benefit was durable, with a 25% improvement in overall survival and 25% of patients alive at three years. The safety profile remained consistent with known immunotherapy and chemotherapy toxicities, with no new safety signals observed.
Tremelimumab for HCC
On October 24, 2022, the U.S. Food and Drug Administration authorized tremelimumab (Imjudo) in combination with durvalumab (Imfinzi) for adults with unresectable hepatocellular carcinoma, the most common form of primary liver cancer. The approved dosing strategy introduced the STRIDE regimen, which consists of a single priming dose of tremelimumab (300 mg) added to durvalumab (1,500 mg), followed by durvalumab every four weeks.
This decision was supported by the HIMALAYA phase III trial, which compared the STRIDE regimen with sorafenib in previously untreated patients with advanced HCC. The combination reduced the risk of death by 22% compared with sorafenib (hazard ratio 0.78). At three years, an estimated 31% of patients treated with Imjudo and Imfinzi were still alive, versus 20% with sorafenib. These landmark survival results were later published in NEJM Evidence in 2022.
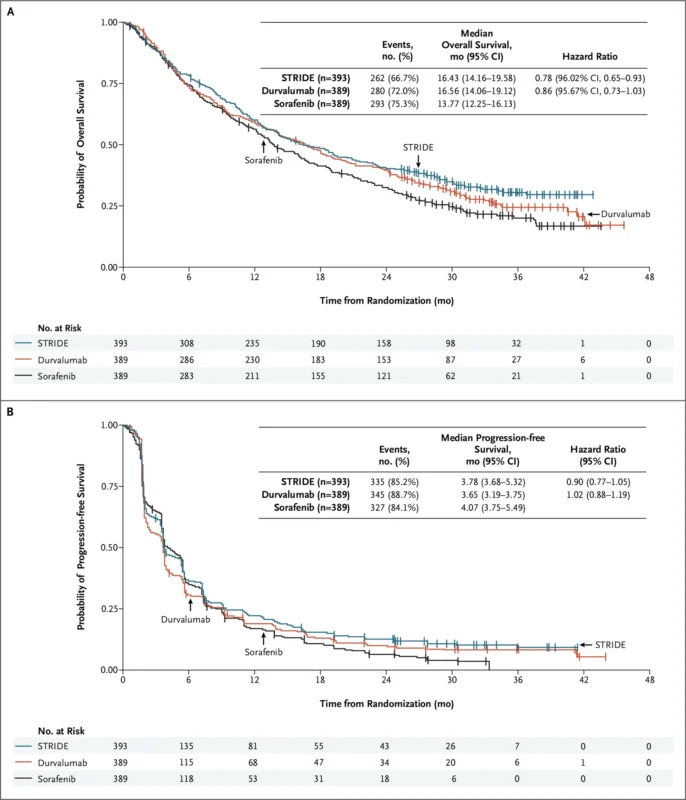
Importantly, the safety profile of the dual immunotherapy regimen was manageable, with no increase in severe liver toxicity or bleeding risk, a critical consideration for patients with underlying cirrhosis. As noted by investigators from Memorial Sloan Kettering Cancer Center, this combination addressed a major unmet need by delivering durable survival benefit without compromising hepatic safety in a fragile patient population.
Side effects of Tremelimumab
Like all immune checkpoint inhibitors, Imjudo works by activating the immune system against cancer, but this same mechanism can also lead to inflammation in healthy tissues. As a result, side effects range from mild and manageable symptoms to less common but potentially serious immune-related reactions. Understanding these effects and how they are managed is essential for ensuring safe and effective treatment.
Common Side Effects
The most frequently reported side effects of tremelimumab, particularly when combined with durvalumab and chemotherapy, include fatigue, diarrhea, nausea, decreased appetite, skin rash, itching, fever, and musculoskeletal pain. Laboratory abnormalities are also common and often detected before symptoms appear. These include elevated liver enzymes (AST, ALT), increased bilirubin, creatinine, and glucose, as well as decreased hemoglobin, lymphocytes, and platelets.
In patients treated for hepatocellular carcinoma, hepatic laboratory disturbances are especially prominent, reflecting both immune-related liver inflammation and the underlying liver disease. In lung cancer, where chemotherapy is added, blood count suppression and kidney function abnormalities occur more often.
Less Common but Serious Side Effects
Less frequently, tremelimumab can trigger severe immune-mediated toxicities that may affect almost any organ system. These include pneumonitis, colitis, hepatitis, nephritis, myocarditis, pancreatitis, and neurologic inflammation. Endocrine glands are also vulnerable, leading to conditions such as hypothyroidism, hyperthyroidism, adrenal insufficiency, hypophysitis, and immune-related diabetes.
Rare but potentially life-threatening complications such as myocarditis, encephalitis, severe skin reactions (including Stevens–Johnson syndrome), and serious ocular inflammation have been reported. Some of these reactions may occur weeks or months after treatment has ended, which is why long-term monitoring remains essential. Infusion-related reactions can also develop during administration and range from mild flushing to severe hypersensitivity.
How Side Effects Are Managed?
Most side effects are managed through early detection, treatment interruption when necessary, and prompt use of corticosteroids for suspected immune-related toxicity. Mild symptoms may respond well to supportive care alone, while moderate to severe immune reactions require immunosuppressive treatment and temporary or permanent discontinuation of therapy.
Patients undergo regular monitoring of liver function, kidney function, blood counts, glucose levels, and thyroid hormones throughout treatment. Hormone replacement therapy is used when endocrine organs are affected. With early recognition and appropriate management, the majority of immune-related side effects are reversible and controllable, allowing many patients to safely continue treatment.
What Is the Recommended Dosage of Tremelimumab?
Tremelimumab (Imjudo) is given by intravenous infusion and is always used in combination with other treatments, most often durvalumab.
For unresectable liver cancer, tremelimumab is given once only as part of the STRIDE regimen. Patients weighing 30 kg or more receive a single 300 mg IV dose, together with durvalumab 1,500 mg IV. Patients weighing under 30 kg receive tremelimumab 4 mg/kg IV once, followed by durvalumab 20 mg/kg. After this first cycle, tremelimumab is stopped, and durvalumab continues alone every four weeks.
For metastatic non–small cell lung cancer, tremelimumab is given together with durvalumab and platinum-based chemotherapy during the initial treatment phase. Patients weighing 30 kg or more receive tremelimumab 75 mg IV, while those under 30 kg receive 1 mg/kg IV, along with durvalumab. This combination is typically given every three weeks for the first four cycles. Afterward, tremelimumab is discontinued, and patients continue with durvalumab-based maintenance therapy.
The exact schedule may vary based on individual factors, and your oncology team will tailor treatment to ensure the best balance between effectiveness and safety.
How is Tremelimumab administered?
Tremelimumab is administered as a 60-minute intravenous infusion through a sterile, low–protein-binding 0.2–0.22 micron in-line filter using a dedicated IV line. It must not be mixed or co-administered with other drugs through the same infusion line and is diluted only in 0.9% sodium chloride or 5% dextrose (D5W). After dilution, the solution is gently inverted to mix and never shaken, and the final concentration must not exceed 10 mg/mL.
When used in combination regimens, tremelimumab is always infused first, followed by durvalumab as a separate 60-minute infusion, and then chemotherapy if prescribed. Each drug requires a separate infusion bag and filter. Patients are observed for at least 60 minutes after the tremelimumab infusion before proceeding with the next agent.
Unopened vials of tremelimumab must be stored refrigerated at 2–8°C in their original carton to protect them from light and must not be frozen or shaken. Once diluted, the solution contains no preservative and should be used immediately when possible. If not administered right away, it may be stored for up to 24 hours either refrigerated at 2–8°C or at room temperature below 30°C. Diluted solutions must also not be frozen or shaken.
What to Avoid During Tremelimumab Treatment?
During treatment, patients are generally advised to avoid medications that suppress the immune system, such as long-term systemic corticosteroids or certain immunosuppressive drugs, unless specifically prescribed to manage side effects. Live vaccines should be avoided due to the risk of altered immune responses.
Alcohol intake is usually limited, particularly in patients with liver cancer, to reduce additional stress on the liver. Herbal supplements and over-the-counter immune boosters should only be used after approval from the oncology team, as they may interfere with immune activity or liver function. Patients are also encouraged to report any new symptoms immediately, rather than self-medicating, to allow early management of immune-related reactions.
Ongoing trials with Tremelimumab
Tremelimumab continues to be actively explored in earlier, potentially curative-stage liver cancer, including a phase I neoadjuvant trial in resectable hepatocellular carcinoma (NCT05701488) led by investigators at Dana-Farber Cancer Institute. This study is evaluating the safety and biological impact of tremelimumab plus durvalumab, given with or without Yttrium-90 selective internal radiotherapy (SIRT) before surgical resection.
Tremelimumab is also being explored in advanced gastrointestinal cancers through the CAMILLA phase I/II trial (NCT03539822), which evaluates cabozantinib plus durvalumab, with or without tremelimumab, in patients with gastric, esophageal, colorectal, and hepatocellular carcinomas. The study focuses on safety, tolerability, and antitumor activity, with overall response rate, progression-free survival, and overall survival as key clinical outcomes. Enrollment is ongoing in the United States, with final study completion expected in 2028.
You can also read about ROWAN Study of TheraSphere with Durvalumab and Tremelimumab in HCC Completed Enrollment on OncoDaily.
The NCT05932199 phase Ib/IIa trial is testing durvalumab + tremelimumab, with or without platinum–pemetrexed chemotherapy, as neoadjuvant treatment for potentially resectable malignant pleural mesothelioma. Patients receive 3 cycles of dual immunotherapy alone or combined with chemotherapy before surgery, followed by up to 12 months of adjuvant durvalumab. The main goal is to achieve >60% recurrence-free survival at 1 year, while also assessing safety, resectability, and pathological response.
The DETECT phase I/IIa trial (NCT06911255) is a single-arm study from Seoul National University Hospital evaluating tremelimumab 300 mg + durvalumab 1500 mg, followed by concurrent TACE and q4w durvalumab, in patients with unresectable HCC (BCLC B–C, Child-Pugh A). The main goal is to assess progression-free survival (PFS) by RECIST 1.1, with secondary endpoints including OS, ORR (RECIST/mRECIST), TTP and safety.
The RESOLVE phase I trial (NCT05154994) from the University of Utah is exploring belinostat (HDAC inhibitor) plus durvalumab, with tremelimumab included in the initial triplet phase, in patients with locally advanced or metastatic/unresectable urothelial carcinoma who are ineligible for or progressing after standard therapy. The study uses a dose-escalation, single-arm design to define the recommended belinostat dose, characterize safety and dose-limiting toxicities, and generate early signals of efficacy (clinical benefit rate, ORR, PFS, OS) under RECIST 1.1.
Find more information on Oncology Drugs.


