Merck announced that the FDA has accepted a priority review for a supplemental Biologics License Application (sBLA) for KEYTRUDA® (pembrolizumab) in treating resectable locally advanced head and neck squamous cell carcinoma (LA-HNSCC). The application seeks approval for KEYTRUDA as a neoadjuvant therapy before surgery, followed by adjuvant treatment with radiotherapy (with or without cisplatin) and then as a single agent. The FDA’s decision is expected by June 23, 2025.
Let’s review what pembrolizumab is and how it works
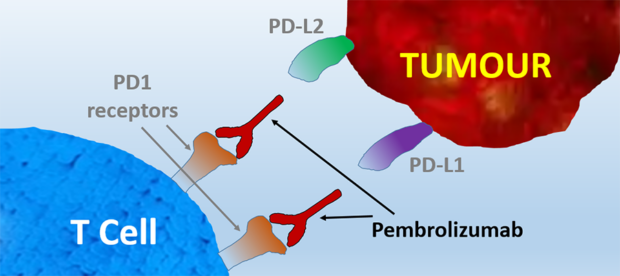
Pembrolizumab is a humanized IgG4 monoclonal antibody that binds to the programmed cell death receptor-1 (PD-1) on activated T cells.
When PD-1 binds to its ligands, PD-L1 and PD-L2 , which are located on tumor cell, T cell activation stops and cancer cells are not recognized by the immune system. Pembrolizumab blocks PD-1 from binding to PD-L1 and PD-L2, which removes the “off” signal on T cells and T cells become activated, helping the immune system fight cancer more effectively.
What is Pembrolizumab Approved For?
The FDA is reviewing KEYTRUDA® (pembrolizumab) for approval as a neoadjuvant treatment before surgery and an adjuvant therapy after surgery in combination with standard-of-care radiotherapy (with or without cisplatin) for resectable locally advanced head and neck squamous cell carcinoma (LA-HNSCC).
What was the previous standard of care for Locally Advanced Head and Neck Squamous Cell Carcinoma?
Until now, the standard treatment for resectable LA-HNSCC has been surgery followed by adjuvant radiotherapy, with or without cisplatin-based chemotherapy depending on risk factors. This approach has remained unchanged for over two decades, highlighting the need for improved treatment options to reduce recurrence and improve survival outcomes.
Clinical Trials and Rationale for Approval
KEYNOTE-689 trial
KEYNOTE-689 is an international, randomized Phase 3 clinical trial evaluating KEYTRUDA as a neoadjuvant treatment followed by adjuvant therapy in combination with standard-of-care radiotherapy, with or without cisplatin, in patients with resectable LA-HNSCC. A key element of the study design is the stratification of efficacy outcomes by programmed death-ligand 1 (PD-L1) combined positive score (CPS) status, which is instrumental in predicting patient response to immunotherapy.
The primary endpoint of the trial is event-free survival (EFS), while key secondary endpoints include overall survival (OS), major pathological response (MPR), pathological complete response (pCR), and safety profiles. The trial enrolled an estimated 704 patients who were randomized in a 1:1 ratio. This design allows for a head-to-head comparison between the experimental arm receiving KEYTRUDA in both neoadjuvant and adjuvant settings and the control arm receiving standard-of-care adjuvant treatment alone.
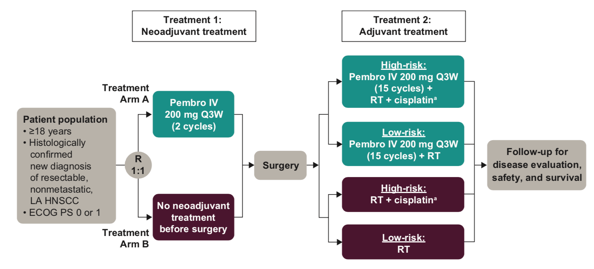
The KEYNOTE-689 trial features an open-label, active-controlled design with two distinct treatment arms
- Experimental Arm:
- Neoadjuvant Phase: Patients receive KEYTRUDA at a fixed dose of 200 mg intravenously (IV) every three weeks (Q3W) for two cycles prior to surgical intervention.
- Adjuvant Phase: Post-surgery, patients are categorized based on risk:
- High-Risk Patients: Receive KEYTRUDA (200 mg IV Q3W for 15 cycles) in combination with standard-of-care radiotherapy plus cisplatin (administered at 100 mg/m² IV Q3W for three cycles).
- Low-Risk Patients: Receive KEYTRUDA (200 mg IV Q3W for 15 cycles) alongside standard-of-care radiotherapy without cisplatin.
- Control Arm:
- No Neoadjuvant Therapy: Patients proceed directly to surgery without preoperative KEYTRUDA.
- Adjuvant Phase: Postoperative treatment is administered based on risk stratification:
- High-Risk Patients: Undergo standard-of-care radiotherapy with cisplatin (100 mg/m² IV Q3W for three cycles).
- Low-Risk Patients: Receive standard-of-care radiotherapy without cisplatin.
This structured approach enables researchers to assess the potential benefits of initiating immunotherapy before surgery (neoadjuvant) in addition to its established use postoperatively (adjuvant). The study also incorporates a clear risk-adapted strategy based on clinical and pathological factors.
Results of Keynote 689 trial
Although final results are pending, KEYNOTE-689 is structured to provide comprehensive data on several key efficacy and safety endpoints:
- Primary Endpoint – Event-Free Survival (EFS): The study’s primary aim is to determine if the addition of KEYTRUDA in the neoadjuvant setting, followed by adjuvant immunotherapy with or without cisplatin, can significantly improve EFS compared to standard adjuvant therapy alone.
- Secondary Endpoints: These include overall survival (OS), the rate of major pathological response (MPR), pathological complete response (pCR), and the overall safety and tolerability of the combined treatment regimen.
- Biomarker Analysis: Outcomes will also be stratified by PD-L1 CPS, allowing for the exploration of potential biomarkers that predict response to immunotherapy in LA-HNSCC
Key Takeaway Messages
- Innovative Treatment Approach: KEYNOTE-689 is at the forefront of exploring the benefits of integrating neoadjuvant and adjuvant immunotherapy with KEYTRUDA in patients with locally advanced HNSCC.
- Potential to Improve Survival: Early intervention with KEYTRUDA, coupled with risk-adapted adjuvant treatment, may enhance event-free survival and overall survival, representing a paradigm shift in managing resectable head and neck cancer.
- Personalized Therapy Based on PD-L1 Status: Stratification by PD-L1 CPS provides a tailored approach, potentially allowing clinicians to better predict which patients will benefit most from immunotherapy.
- Robust Clinical Data: The trial’s large-scale, randomized design and comprehensive evaluation of clinical endpoints promise to deliver high-quality evidence that could redefine standard treatment protocols.
- Enhanced Safety Profile: The integration of KEYTRUDA with standard radiotherapy (and cisplatin where appropriate) is being carefully monitored for safety, ensuring that any improvements in efficacy do not come at the expense of increased toxicity.
KEYNOTE-689 represents a significant step forward in the management of locally advanced head and neck squamous cell carcinoma. By incorporating KEYTRUDA as both a neoadjuvant and adjuvant therapy, the trial seeks to leverage the immunomodulatory benefits of PD-1 inhibition at an earlier stage of treatment. This approach is poised to address the high recurrence rates and suboptimal outcomes associated with standard-of-care therapies in LA-HNSCC.
What is a Biologics License Application (sBlA)?
A Biologics License Application (BLA) is a request submitted to the U.S. Food and Drug Administration (FDA) to approve a biologic drug—such as monoclonal antibodies, vaccines, or cell therapies—for use in patients. The application includes data from laboratory studies and clinical trials demonstrating the drug’s safety, efficacy, manufacturing process, and quality control measures.
A supplemental Biologics License Application (sBLA) is filed when the manufacturer wants to make changes to an already approved biologic. These changes can include new indications (expanding the drug’s use for additional diseases or patient populations), new dosing regimens, updated safety information, or modifications to the manufacturing process. The FDA reviews sBLAs to ensure that the proposed changes maintain the drug’s safety and effectiveness.
A New Era of Treatment for Locally Advanced Head and Neck Squamous Carcinoma
The FDA’s acceptance of the sBLA for pembrolizumab marks a significant step forward in the treatment of resectable locally advanced head and neck squamous cell carcinoma (LA-HNSCC). The KEYNOTE-689 trial is the first Phase 3 study to demonstrate a meaningful improvement in event-free survival using an anti-PD-1 therapy in both neoadjuvant and adjuvant settings, potentially changing the standard of care for these patients.
Future research will likely explore pembrolizumab in combination with novel immunotherapies, targeted agents, or alternative chemotherapy regimens to enhance efficacy. Additionally, ongoing trials may assess its role in even earlier stages of disease, aiming to further reduce recurrence rates and improve long-term outcomes.
Written by Sona Karamyan, MD


