Nirogacestat is an FDA-approved oral therapy for adults with progressing desmoid tumors, a rare type of soft tissue tumor. Approved in November 2023, Ogsiveo became the first targeted treatment available for this challenging condition. This article will help you understand what Nirogacestat is, how it works, what to expect during treatment, and how it may benefit patients both now and in the future.
What Is Nirogacestat and How Does It Work?
Nirogacestat, marketed under the name Ogsiveo, is a targeted therapy designed to block a protein complex called gamma-secretase. This complex is responsible for activating certain cellular signals, particularly the Notch signaling pathway, which helps some tumors grow and spread.
By inhibiting gamma-secretase, Nirogacestat stops the release of signals that promote tumor growth. This is especially important in desmoid tumors, where abnormal Notch signaling can drive tumor progression. The drug may also boost the effectiveness of other treatments by preserving important targets on tumor cells, such as BCMA in certain blood cancers.
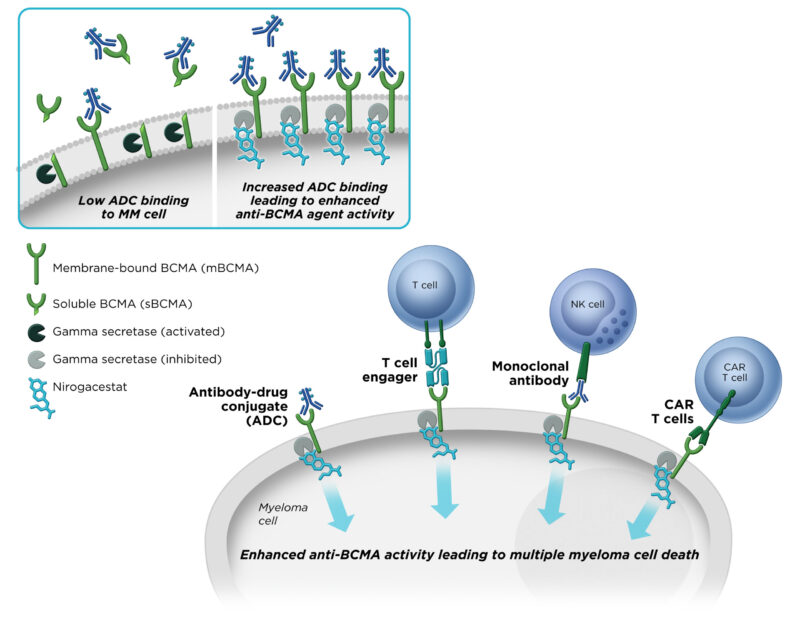
Photo from SpringWorks Therapeutics Official Website
What Type of Cancer Does Ogsiveo Treat?
Ogsiveo is specifically approved for adults with progressing desmoid tumors who need systemic therapy. Desmoid tumors, though non-metastatic, can be locally aggressive and cause pain, limited mobility, and organ dysfunction.
How Effective Is Nirogacestat?
Ogsiveo’s approval was based on the Phase 3 DeFi clinical trial, with final results published in The New England Journal of Medicine in March 2023. In this study, 142 patients with progressing desmoid tumors were randomized to receive either Nirogacestat or a placebo.
Nirogacestat was approved based on a major international study called the DeFi trial. This Phase 3 clinical trial included 142 adults with progressing desmoid tumors. Some received Ogsiveo, while others got a placebo (a pill with no active drug). The study showed that Ogsiveo helped patients live longer without their tumor growing—76% were progression-free after two years compared to 44% in the placebo group.
About 41% of patients saw their tumors shrink on Ogsiveo, and 7% had their tumors disappear completely. These responses often began around 5 to 6 months into treatment. Patients also reported less pain, fewer symptoms, and better physical function and quality of life.
Most side effects were mild to moderate. The most common included diarrhea, nausea, tiredness, low phosphate levels, and skin rash. A specific concern for women of childbearing age was ovarian dysfunction, which happened in 75% of those taking Ogsiveo—but most recovered during or after treatment.

Learn more about Radiotherapy For Soft Tissue Sarcomas: Types, Success Rate, Side Effects on OncoDaily.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Ogsiveo was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
Long-Term Effectiveness: New Data from 2025
New results from a major study presented at the ESMO Sarcoma and Rare Cancers 2025 Congress show that Nirogacestat continues to help patients with desmoid tumors even after several years of treatment.
In this study, patients took Ogsiveo for up to four years. Most stayed on treatment for at least one year, and about one in five continued for four years. The longer patients stayed on treatment, the more their tumors responded—partial and complete shrinkage became more common over time.
Many patients also felt better physically and emotionally during treatment. They reported less pain, improved daily functioning, and a better overall quality of life. Side effects remained consistent with earlier reports and became less frequent after the first year, with very few people needing to stop treatment due to side effects after year two.
These long-term findings confirm that Ogsiveo not only controls tumor growth but also helps patients feel better for years, with side effects that are generally manageable.
What Is the Recommended Dose of Ogsiveo?
Nirogacestat is available as a 50 mg tablet and is prescribed for adults with growing desmoid tumors. The usual recommended dose is 150 mg (three tablets) taken twice a day by mouth. Treatment continues as long as it helps and side effects remain manageable.
If certain side effects occur—like severe diarrhea, high liver enzymes, or low phosphate or potassium levels—the doctor may pause treatment. Once symptoms improve, the medication can often be restarted at a lower dose. No dose adjustment is needed for patients with mild or moderate kidney issues. However, information is limited for people with serious liver problems.
How Should Nirogacestat Be Taken?
Ogsiveo is taken by mouth and can be taken with or without food. Tablets must be swallowed whole and should not be chewed, crushed, or broken. If a dose is missed or vomited, the next dose should be taken at the usual time—there’s no need to make up for the missed dose. Ogsiveo tablets should be kept at room temperature, ideally between 20°C and 25°C. Short-term exposure to temperatures between 15°C and 30°C is considered acceptable.
Nirogacestat Side Effects and Management
Nirogacestat, like many cancer treatments, can cause side effects. Most are manageable, but knowing what to expect helps patients stay comfortable and continue treatment safely.
Common Side Effects
The most common side effects include diarrhea, nausea, tiredness, and skin issues like rashes or mouth sores. Blood tests may also show low phosphate or potassium levels, or high liver enzymes. For women of childbearing age, Ogsiveo may affect the ovaries, sometimes leading to missed periods or signs of early menopause.
Rare or Serious Side Effects
Less common side effects include high liver enzyme levels, certain types of skin cancer (not melanoma), and rare allergic reactions like swelling or rash. Infections and fever may also happen, though not often. So far, no major effects have been seen on male fertility, but ovarian changes in women remain an important concern.
How Are Side Effects Managed?
Doctors closely monitor patients during treatment. Common issues like diarrhea or nausea are treated with fluids, diet changes, and medications. Fatigue is addressed by improving sleep and nutrition. Blood tests help check for changes in electrolytes or liver function, and problems are managed with supplements or dose changes.
For women who may want children in the future, fertility preservation should be discussed before starting Ogsiveo. Skin issues are treated with creams or mouth rinses, and sometimes the dose is adjusted. Since there is a small risk of skin cancer, regular skin checks are recommended during long-term treatment.

What Should You Avoid During Treatment?
While taking Ogsiveo, avoid grapefruit, Seville oranges, and starfruit, as they may interfere with the drug’s metabolism. Also, avoid proton pump inhibitors and H2 blockers, which can reduce the effectiveness of the medication. If antacids are needed, they should be taken two hours before or after your dose. Always tell your healthcare provider about any medications or supplements you’re taking.
How Long Does Ogsiveo Stay in the Body?
Nirogacestat is metabolized mainly by the liver enzyme CYP3A4, and smaller contributions from other enzymes. It stays in the body for about 23 hours, and is mostly eliminated through stool, with small amounts leaving the body through urine and breath.
Looking Ahead: The Future of Ogsiveo in Cancer Treatment
With its strong performance in desmoid tumors, Nirogacestat is opening new doors for patients with rare cancers. As clinical trials continue, researchers hope it will become part of combination strategies in other cancers, particularly blood cancers and solid tumors where gamma-secretase plays a role. If you’re facing desmoid tumors or are interested in clinical trials with Nirogacestat, speak with your oncologist. You may qualify for this promising new therapy or be eligible to participate in research that could benefit others in the future.
Ongoing Research and New Trials
A new trial led by M.D. Anderson Cancer Center is exploring why some patients respond better than others to Nirogacestat. Researchers are looking at MRI scans, tumor tissue, blood tests, and genetic markers to learn how to improve treatment selection. They are also studying ovarian side effects and how dose levels may impact fertility.
Nirogacestat is also being tested in multiple myeloma, often in combination with other therapies, as it may enhance the activity of drugs that target the BCMA protein on myeloma cells.
If you’re a healthcare provider, access the professional version here.


