Toripalimab (Loqtorzi) is a PD-1 inhibitor, a type of immune checkpoint inhibitor that works by blocking the PD-1 pathway, which cancer cells use to evade the immune system. By inhibiting PD-1, toripalimab helps the immune system recognize and attack cancer cells.
It was first approved in China in 2018 for the treatment of unresectable or metastatic melanoma. In 2023, toripalimab received FDA approval for use in nasopharyngeal carcinoma (NPC), initially in combination with cisplatin and gemcitabine as first-line therapy for recurrent or metastatic NPC. It was also approved as monotherapy for patients with NPC who have progressed after platinum-based chemotherapy.
What Is Toripalimab and How Does It Work?
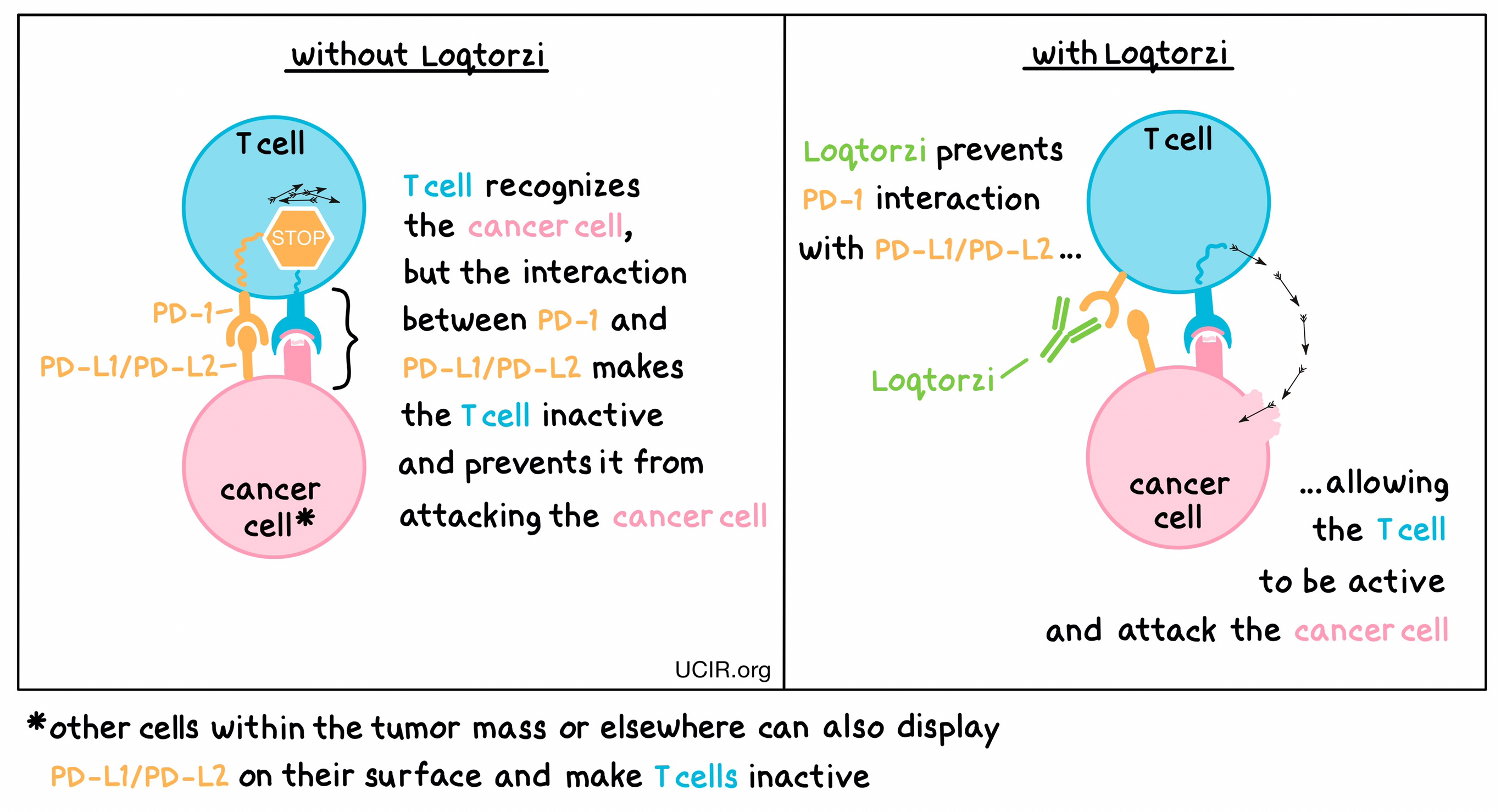
Loqtorzi is an immunotherapy drug that belongs to a class of medications called PD-1 inhibitors. Normally, the immune system uses T-cells to detect and destroy abnormal or cancerous cells. However, cancer cells can develop mechanisms to evade immune detection, including the PD-1 pathway. PD-1 is a checkpoint protein on T-cells that, when activated by its ligands (PD-L1/PD-L2) on tumor cells, sends a signal to turn off the T-cells, preventing them from attacking the cancer.
By blocking the interaction between PD-1 and its ligands, toripalimab prevents this “off” signal, allowing T-cells to stay active and continue targeting and destroying cancer cells.
What Cancers Does Toripalimab Treat?
Loqtorzi was first approved in China in December 2018 for metastatic melanoma. It was later approved for nasopharyngeal carcinoma (NPC). In October 2023, the FDA approved Loqtorzi in the U.S. for NPC treatment, both in combination with chemotherapy as a first-line treatment and as monotherapy for patients with disease progression after platinum-based chemotherapy.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Toripalimab was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it is both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
Efficacy and Results from Clinical Trials
The approvals of Loqtorzi were based on pivotal clinical trials, including the JUPITER-02 study and the POLARIS-02 study. In the JUPITER-02 study, toripalimab combined with gemcitabine-cisplatin (GP) was established as a new standard of care for recurrent or metastatic nasopharyngeal carcinoma (NPC). The results of this study demonstrated a progression-free survival (PFS) of 11.7 months compared to 8.0 months for chemotherapy alone (HR = 0.52, p = 0.0003).
In terms of overall survival (OS), the toripalimab arm did not reach OS, while the placebo arm showed an OS of 33.7 months (HR = 0.63, p = 0.0083). At the three-year mark, OS rates were 64.5% in the Loqtorzi arm versus 49.2% in the placebo arm. The study also highlighted an increase in immune-related adverse events, with 54.1% of patients in the toripalimab group experiencing them, compared to 21.7% in the placebo group.
The POLARIS-02 study evaluated Loqtorzi in pretreated NPC patients. This study found an objective response rate (ORR) of 20.5%, a median duration of response (DOR) of 12.8 months, and an overall survival (OS) of 17.4 months. Notably, patients who showed a ≥50% drop in plasma Epstein-Barr virus (EBV) DNA by day 28 had significantly better outcomes, with an ORR of 48.3% compared to 5.7% in those without such a drop (p = 0.0001). These results further demonstrated toripalimab’s potential to offer durable responses in patients with recurrent or metastatic NPC, particularly those with favorable biomarkers.
Learn more about Immunotherapy for Cancer on OncoDaily.
Ongoing trials with Toripalimab
Toripalimab is being investigated in various cancers:
Ongoing clinical trials are exploring new ways to use Loqtorzi in treating different cancers. One Phase II study is investigating whether combining toripalimab with another immunotherapy drug, 9MW2821, is more effective than using 9MW2821 alone in patients with advanced or metastatic urothelial carcinoma who have not yet received systemic treatment.
Another trial is focused on hepatocellular carcinoma (HCC), the most common type of liver cancer. This Phase Ib study is assessing the safety and effectiveness of combining Loqtorzi with JS014 and a targeted procedure called transarterial chemoembolization (TACE). Researchers hope this combination will enhance the immune system’s ability to fight the tumor.
Additionally, a Phase II trial is evaluating toripalimab in esophageal squamous cell carcinoma. Patients in this study receive toripalimab alongside chemotherapy and radiation before undergoing surgery. Researchers are monitoring how well the treatment shrinks tumors before surgery and how it impacts long-term survival. These studies aim to expand treatment options and improve outcomes for patients with these cancers.
Read about What Are the Signs That Immunotherapy Is Working? on OncoDaily.
What to Expect During Treatment?
During treatment with Loqtorzi, patients can expect to receive the medication through an intravenous (IV) infusion, which typically lasts between 30 to 60 minutes. The treatment schedule varies depending on the specific cancer being treated and the doctor’s recommendations, with infusions usually given every two or three weeks. Throughout the course of treatment, regular monitoring is essential to track progress and detect any potential side effects early.
Patients will undergo routine blood tests to check organ function and immune system activity, as well as imaging scans such as CT or MRI to assess how well the treatment is working. By closely following this schedule and attending all follow-up appointments, patients and their healthcare teams can ensure the best possible outcomes while managing any side effects that may arise.
Toripalimab Side Effects and Management
Loqtorzi is an immunotherapy used to treat cancers like nasopharyngeal carcinoma (NPC) by helping the immune system fight cancer cells. While it can significantly improve outcomes, it may also cause side effects that need careful monitoring and management.
Common Side Effects
Many patients experience mild to moderate side effects, such as fatigue, low thyroid hormone levels (hypothyroidism), and muscle or joint pain. Digestive issues like nausea and constipation are also common. Some people develop skin reactions (such as rashes), fever, or tingling and numbness in the hands and feet (peripheral neuropathy). Other possible effects include coughing, mild respiratory infections, headaches, and dizziness.
Serious Side Effects
In some cases, toripalimab can cause the immune system to mistakenly attack healthy organs, leading to serious conditions. These include pneumonitis (lung inflammation, causing breathing difficulties), colitis (inflammation of the bowel, leading to diarrhea and abdominal pain), and hepatitis (liver inflammation, which can cause yellowing of the skin or fatigue). Hormonal imbalances due to pituitary gland inflammation (hypophysitis) or other endocrine problems may also occur. Less commonly, toripalimab can lead to kidney inflammation (nephritis), severe skin reactions, heart inflammation (myocarditis), or even neurological symptoms like confusion or seizures. These require immediate medical attention.
How Are These Side Effects Managed?
Doctors monitor patients closely with regular blood tests to check thyroid, liver, and kidney function. If mild side effects occur, they may be managed with supportive care such as hydration, symptom relief medications, or thyroid supplements. For more severe immune-related side effects, steroids or other immunosuppressive drugs may be needed to calm the immune system. In some cases, treatment may need to be paused or adjusted.
Why Early Detection Matters?
Recognizing symptoms early and reporting them to a doctor can prevent complications and ensure treatment remains effective. With careful monitoring and timely management, patients can continue their therapy while minimizing risks, allowing them to benefit from toripalimab’s cancer-fighting potential.
Long-Term Outlook with Toripalimab
Toripalimab has shown durable responses in NPC and other cancers. While it is not a cure, it helps prolong survival and improve quality of life for many patients. Ongoing trials aim to refine its effectiveness and expand its indications.
What to Avoid During Toliparimab Treatment?
During treatment with toripalimab, patients should take certain precautions to ensure the best possible outcomes and minimize risks. Some antibiotics, such as cefepime and ceftriaxone, may interact with the immune system or affect how the body responds to immunotherapy, so their use should be carefully considered and discussed with a doctor. Additionally, live vaccines should be avoided during treatment, as they can pose a higher risk of infection due to the way toripalimab affects the immune system.
Patients should also be cautious with alcohol and other immunosuppressive drugs, as these can interfere with the body’s ability to fight infections and may increase the risk of side effects. It is important to consult a healthcare provider before making any changes to medications, vaccinations, or lifestyle habits to ensure a safe and effective treatment plan.
Real-World Effectiveness
Clinical trials have demonstrated toripalimab’s strong efficacy, and real-world data continue to support its benefit in cancer treatment. Patients should discuss their individual prognosis and treatment options with their oncologist.
Looking Ahead – The Future of Toripalimab
Toripalimab’s role in cancer treatment is expanding, with ongoing studies evaluating new cancer types, combination therapies, and improved dosing schedules. It represents a promising option for patients needing advanced immunotherapy solutions.
If you’re a healthcare provider, access the professional version here.