Enfortumab vedotin, also known as Padcev, is a groundbreaking antibody-drug conjugate (ADC) designed to treat urothelial carcinoma, the most common type of bladder cancer. It has received FDA approval for patients with advanced bladder cancer who have already undergone standard therapies, as well as for use in combination with pembrolizumab as a first-line treatment.
If you’re a healthcare provider, access the professional version here.
What Is Enfortumab Vedotin and How Does It Work?
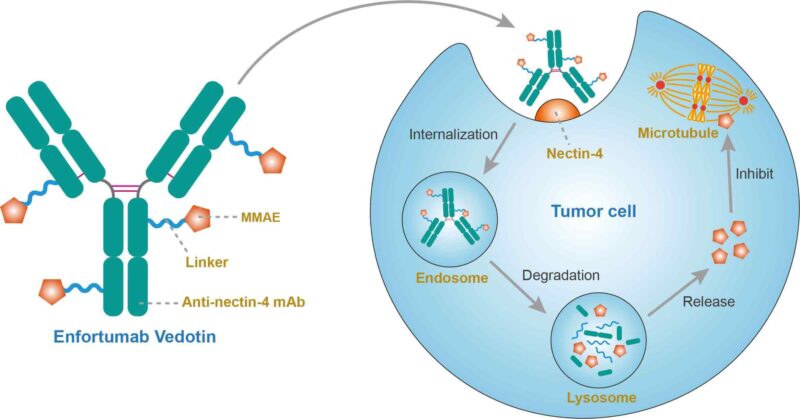
Enfortumab vedotin (Padcev) is an innovative cancer therapy that combines a monoclonal antibody with a potent chemotherapy agent. It specifically targets Nectin-4, a protein found in high levels on cancer cells. Once the drug binds to Nectin-4, it is absorbed by the cancer cell, releasing monomethyl auristatin E (MMAE), which disrupts cell division and causes tumor cell death. This targeted approach minimizes damage to healthy cells, reducing side effects compared to traditional chemotherapy.
- Targeting Cancer Cells – The drug is designed to find a protein called Nectin-4, which is found in large amounts on bladder cancer cells (urothelial carcinoma). It works like a key fitting into a lock, allowing the drug to attach to the cancer cell.
- Getting Inside the Cancer Cell – Once enfortumab vedotin attaches to the cancer cell, it is pulled inside, like a package being delivered inside a building.
- Releasing the Chemotherapy – Inside the cancer cell, the drug releases a powerful chemotherapy agent called monomethyl auristatin E (MMAE). This substance disrupts the cancer cell’s ability to divide and grow.
- Cancer Cell Death – Since the cancer cell can no longer multiply, it eventually dies, stopping or slowing the cancer’s progression.
What makes enfortumab vedotin special is its precision. Because it specifically targets cancer cells that have Nectin-4, it helps limit damage to normal cells. This approach improves effectiveness while reducing some of the severe side effects often seen with traditional chemotherapy. As a result, enfortumab vedotin has become an important treatment option for patients with advanced bladder cancer, especially those who have already tried other therapies.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Padcev was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
What Cancers Does Enfortumab Vedotin Treat?
The FDA has approved enfortumab vedotin for urothelial carcinoma, particularly in patients who:
Have previously received a PD-1/PD-L1 checkpoint inhibitor and platinum-based chemotherapy.
Are ineligible for cisplatin-based chemotherapy, in combination with pembrolizumab as a first-line treatment.
Clinical Trials and Effectiveness
Several clinical trials have established enfortumab vedotin as a superior treatment for advanced bladder cancer:
- EV-201 Trial (2019, JCO): A Phase II study that showed a 44% objective response rate in patients who had exhausted other treatments, with 12% achieving complete remission.
- EV-301 Trial (2021, NEJM): A Phase III study that demonstrated significant survival benefits over chemotherapy, improving overall survival (12.9 vs. 9.0 months) and progression-free survival (5.6 vs. 3.7 months).
- EV-103 Cohort K (2023, JCO): Showed that enfortumab vedotin combined with pembrolizumab had a 64.5% response rate, with many patients experiencing durable responses.
- EV-302 Trial (2024, NEJM): Established enfortumab vedotin + pembrolizumab as a new standard of care, reducing disease progression or death risk by 55%, with a 31.5-month overall survival.
Ongoing Research and Future Applications
Ongoing trials are exploring enfortumab vedotin in other cancers, including:
Head and Neck Cancer (EV-202 Study): A Phase II trial showing a 23.9% response rate in patients with previously treated metastatic head and neck cancer.
Colorectal and Liver Cancer (New Phase II Study): Evaluating its effectiveness in patients with advanced colorectal and hepatocellular carcinoma who have undergone prior treatments.
FDA Approval and Its Impact on Patients
The FDA’s approval of enfortumab vedotin provides new hope for patients with advanced bladder cancer. Its approval signifies that it has passed rigorous testing for safety and effectiveness, allowing more patients access to an advanced therapy that significantly improves survival and quality of life.
How is Enfortumab Vedotin administered?
Enfortumab vedotin (Padcev) is given through an IV infusion over 30 minutes. It is usually given on specific days of a treatment cycle, either alone or with pembrolizumab. When combined, Padcev is given first. Treatment continues until the cancer worsens or side effects become too severe. The dose may be adjusted based on how a patient responds. Padcev must be stored in the refrigerator and handled carefully before use. It should not be frozen, shaken, or given as a quick injection.
Common Side Effects and Management
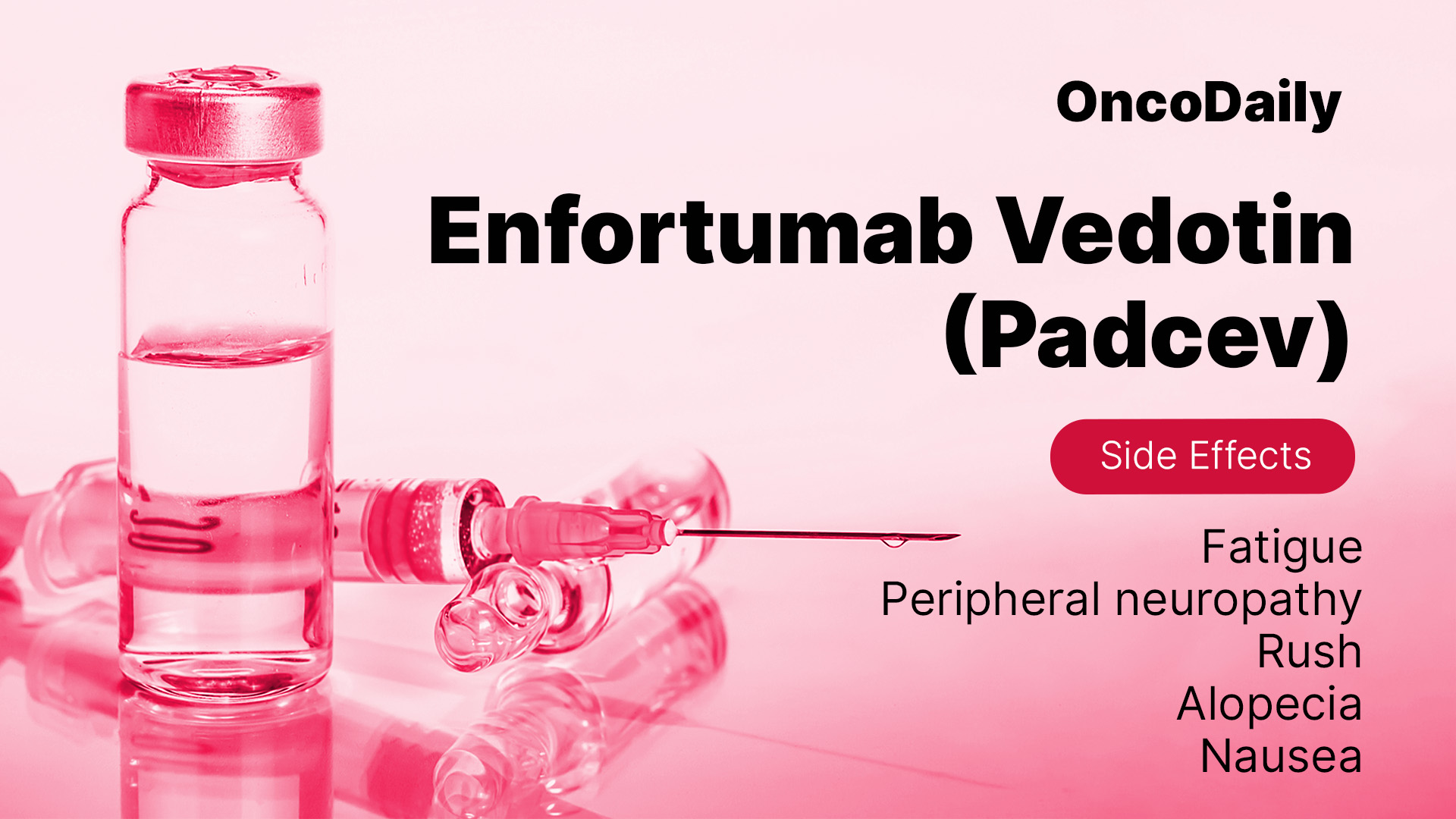
While enfortumab vedotin is effective, it can cause side effects, including:
- Fatigue – Managed with rest and hydration.
- Peripheral Neuropathy – Tingling, numbness, or pain in hands and feet, requiring dose adjustments if severe.
- Skin Reactions – Rashes, redness, or peeling, managed with skincare routines and medications.
- Hair Loss (Alopecia) – A temporary effect that resolves after treatment ends.
- Gastrointestinal Issues – Nausea, vomiting, or diarrhea, managed with supportive medications.
Serious Side Effects
- Severe Skin Reactions – Blistering and peeling require immediate medical attention.
- Lung Issues – Shortness of breath or persistent cough should be reported immediately.
- Hyperglycemia – Blood sugar levels must be monitored closely, especially in diabetic patients.
What Should You Avoid During Treatment?
- Missed Doses – It’s crucial to follow the treatment schedule for maximum effectiveness.
- Infections – Patients should practice good hygiene and avoid contact with sick individuals.
- Grapefruit and Grapefruit Juice – These can interfere with drug metabolism.
- Live Vaccines – Avoid as they can pose risks due to a weakened immune system.
- Pregnancy and Breastfeeding – Enfortumab vedotin may harm an unborn baby; contraception is recommended during and after treatment.
- Sun Exposure – Increased risk of skin reactions; use sunscreen and protective clothing.
Long-Term Effectiveness
Enfortumab vedotin has shown long-term benefits, particularly when combined with pembrolizumab. Clinical trials have demonstrated prolonged survival, with 31.5 months overall survival in the EV-302 trial, making it one of the most effective treatments for advanced urothelial carcinoma.
Looking Ahead: The Future of Treatment
With ongoing research, enfortumab vedotin is being tested in other cancers, new drug combinations, and earlier treatment settings. As studies progress, it may expand into additional cancer types, offering hope to even more patients in need.
Enfortumab vedotin (Padcev) represents a major advancement in cancer treatment, particularly for bladder cancer. With its targeted approach, high response rates, and potential in multiple cancers, it provides new hope for patients with advanced disease. If you or a loved one is considering this treatment, consult with an oncologist to understand the benefits, risks, and best treatment plan tailored to your needs.
Read more about Targeted Therapy vs Immunotherapy: Which Is Right for You, Key Differences & Similarities on OncoDaily.
Written by Mariam Khachatryan, MD