Dordaviprone is a new type of cancer treatment that has been making headlines in the oncology community. In August 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval for its use in treating a rare and aggressive brain cancer called recurrent H3 K27M-mutant diffuse midline glioma in adults and children aged one year and older whose disease has progressed after previous therapy.
This approval represents a major milestone, as it marks the first systemic therapy available for this devastating form of brain cancer. Patients and families who have faced limited treatment options now have a new possibility for hope. Dordaviprone’s unique way of targeting cancer cells sets it apart from other drugs, and ongoing research suggests it could also be helpful in other hard-to-treat tumors.
What Is Dordaviprone and How Does It Work?
Dordaviprone is a first-in-class small-molecule drug from a group of compounds known as imipridones. Dordaviprone (Modeyso) was first developed by Oncoceutics, advanced by Chimerix, and later acquired by Jazz Pharmaceuticals, evolving from an early experimental therapy to a near-commercial cancer treatment with multiple regulatory designations. Unlike many chemotherapy drugs that kill cells indiscriminately, dordaviprone works through a dual mechanism that specifically targets vulnerabilities in cancer cells while sparing most healthy tissue.
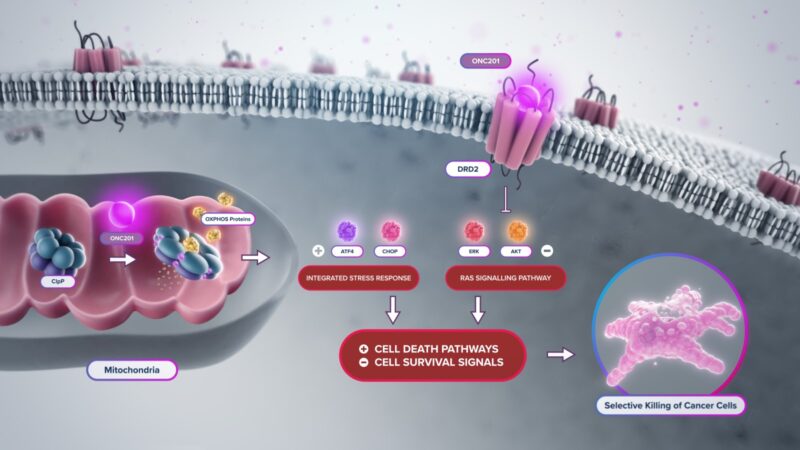
At the molecular level, dordaviprone works in two main ways:
- Mitochondrial stress induction – The drug binds to and activates a protein called mitochondrial caseinolytic protease P (ClpP) inside tumor cells. This enzyme is responsible for breaking down damaged proteins within mitochondria. When dordaviprone over-activates ClpP, it causes uncontrolled breakdown of mitochondrial proteins, leading to energy disruption, stress, and eventual death of the cancer cell.
- Blocking cancer cell signaling – Dordaviprone also blocks the dopamine receptor D2 (DRD2), which is abnormally active in several cancers, including H3 K27M-mutant diffuse midline gliomas. This receptor is involved in signaling pathways (like PI3K/AKT and MAPK) that cancer cells use to survive and grow. Blocking DRD2 disrupts these survival signals, making cancer cells more vulnerable.
This two-pronged attack—cutting off the cell’s energy supply and blocking its growth signals—makes dordaviprone especially effective against tumors that depend heavily on mitochondria for survival.
Source: Chimerix Official Website, a part of Jazz Pharmaceuticals
What Cancers Does It Treat?
As of its FDA approval in August 2025, dordaviprone is approved only for the treatment of:
- Recurrent H3 K27M-mutant diffuse midline glioma (DMG) in patients aged ≥1 year who have experienced disease progression after prior therapy.
This mutation occurs in tumors that develop in critical brain and spinal cord regions such as the thalamus, brainstem, and spinal cord midline structures. The H3 K27M mutation changes the way tumor cells regulate DNA and gene expression, making the cancer more aggressive and resistant to traditional treatments like surgery, radiation, or chemotherapy.
While the current approval is specific to this mutation in midline gliomas, researchers are also investigating dordaviprone for other brain and solid tumors.
Efficacy and Results from Clinical Trials
The FDA’s approval was based on an integrated analysis of 50 patients treated across five early-stage, open-label U.S. trials—ONC006, ONC013, ONC014, ONC016, and ONC018.
Key findings from the trials:
- Overall response rate (ORR): 22% (95% CI: 12–36)
- Median duration of response (DOR): 10.3 months (95% CI: 7.3–15.2)
- Durability:
73% of responders maintained their response for at least 6 months
27% of responders maintained their response for over 12 months
These results are notable because recurrent H3 K27M-mutant DMG historically responds poorly to available treatments, with most therapies offering only short-term benefit. The fact that some patients experienced a year or more of tumor control represents a meaningful improvement.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Modeyso was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
Dordaviprone Side Effects and How to Manage Them?
Dordaviprone is an important new treatment for certain aggressive brain tumors, but like all cancer medicines, it can cause side effects. Understanding what these side effects are, how they might feel, and what can be done to manage them is an important part of treatment. Many people take the drug without serious problems, but some may experience symptoms that need medical attention. Knowing what to expect helps you and your care team act quickly if problems arise.
Common Side Effects
The most frequent side effects seen in clinical studies included nausea, vomiting, and diarrhea. These stomach-related symptoms can range from mild to bothersome, but they are often manageable with medicines that prevent nausea, changes in diet, and staying well hydrated. Fatigue is another common symptom and can be caused by both the medicine and the cancer itself.
Headaches are sometimes reported and usually improve with standard pain relief, as long as your doctor agrees it is safe. Some people develop mild changes in liver blood tests, which is why your doctor will check your liver function regularly during treatment. Changes in blood counts, such as mild anemia or lower platelets, can also occur but are usually temporary and respond to supportive care.
Less Common Side Effects
Some people may develop allergic or hypersensitivity reactions, which can show up as a rash, itching, or—rarely—more serious swelling called angioedema. Another possible but less common issue is a change in the heart’s electrical activity, called QTc prolongation. This is usually detected through an ECG (electrocardiogram) and is more likely if you already have heart problems or are taking other medicines that affect the heartbeat. Dordaviprone can also harm an unborn baby, so it is essential to avoid pregnancy during treatment and for some time afterward. Your doctor will discuss safe and effective birth control options before you start therapy.
Managing Side Effects
The key to managing side effects is early recognition. For nausea, vomiting, or diarrhea, your care team may give you anti-sickness tablets or recommend dietary changes and extra fluids. Liver function will be checked with regular blood tests, and the dose may be adjusted if necessary. If you are at risk for heart rhythm changes, your doctor may order ECGs before and during treatment and avoid combining the drug with others that affect the QTc interval.
Allergic reactions are treated depending on severity, ranging from antihistamines for mild symptoms to stopping treatment if the reaction is serious. For all patients, ongoing conversations with your care team about symptoms—no matter how small they may seem—are important to keep treatment on track.

What is Recommended Dosage?
Dordaviprone comes in 125 mg capsules. For most adults, the recommended dose is 625 mg taken once a week. Treatment continues as long as the tumor is controlled and side effects remain manageable. If serious side effects occur, treatment may be paused, the dose lowered, or in some cases, stopped completely.
How the Medicine Is Taken?
Dordaviprone is taken by mouth, once a week, on an empty stomach—either at least one hour before eating or three hours after. Taking it at the same time each week helps maintain steady levels in the body. Capsules should be swallowed whole. For people who cannot swallow capsules, the contents can be mixed with a small amount of liquid and taken immediately.
The same liquid preparation can be given through a feeding tube. If a dose is missed but less than two days have passed, take it as soon as possible. If more than two days have passed, skip the missed dose and take the next one on schedule. If vomiting occurs after taking the medicine, do not take an extra dose.
What to Avoid During Treatment?
Some things can interfere with dordaviprone’s safety and effectiveness. Medicines that strongly affect the liver enzyme CYP3A4 can change the way the drug is broken down, increasing the risk of side effects. Drugs that also prolong the QTc interval can increase heart rhythm problems. Grapefruit and grapefruit juice should be avoided because they can raise the amount of dordaviprone in your blood. Because of the risk to an unborn baby, pregnancy must be avoided during treatment and for a period afterward. Treatment should never be stopped suddenly without talking to your doctor, as this could allow the cancer to progress.

You can also read about Glioblastoma Cure Rate: What Patients Should Know on OncoDaily.
How Long the Drug Stays in the Body?
Dordaviprone is processed mainly in the liver and has a half-life of about 11 hours, meaning it takes this long for the amount in your body to drop by half. Even so, because of its unique activity, it is only taken once a week. Most of the drug leaves the body through the urine.
Effectiveness Over Time
In clinical studies, many patients whose tumors responded to dordaviprone were able to keep the cancer under control for more than six months. Some patients experienced benefits for over a year, which is a significant improvement for this type of aggressive brain cancer. While the medicine is not a cure, it can offer valuable time and better quality of life for some people.
What Other Trials Are Ongoing?
Even though dordaviprone is currently approved only for recurrent H3 K27M-mutant DMG, researchers are exploring its potential in other settings.
- ACTION Phase 3 Trial – This global, randomized, double-blind study is testing dordaviprone in newly diagnosed H3 K27M-mutant diffuse glioma after radiation therapy. The goal is to see if earlier use can extend survival and delay disease progression.
- ONC201 in Refractory Meningioma – A University of Nebraska–sponsored pilot trial is studying dordaviprone in patients with hard-to-treat meningiomas. This includes patients receiving it before surgery to measure drug levels in tumor tissue, as well as those with unresectable or recurrent disease.
- Other tumor types – Early research is assessing its use in other brain tumors and possibly solid tumors with high mitochondrial dependency or DRD2 overexpression.
If you’re a healthcare provider, access the professional version here.