Darolutamide is a medicine used to treat certain types of prostate cancer. It works by blocking male hormones that help prostate cancer grow. Darolutamide has been approved by the U.S. Food and Drug Administration (FDA) for different stages of advanced prostate cancer, giving many patients a new hope in their fight against the disease.
What Is Darolutamide and How Does It Work?
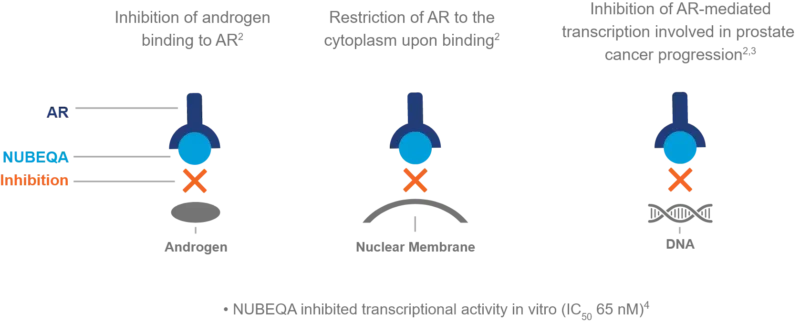
Darolutamide, sold under the brand name Nubeqa, belongs to a group of drugs called androgen receptor inhibitors. Androgens, like testosterone, are male hormones that can make prostate cancer cells grow faster. Darolutamide works by blocking the androgen receptors on cancer cells, preventing these hormones from attaching and sending growth signals.
Because of this, darolutamide helps slow down or stop cancer from growing. It also produces an active form called keto-darolutamide which helps fight the cancer even more. Unlike some similar drugs, darolutamide has less effect on the brain, which means it may cause fewer side effects like fatigue or dizziness.
What Types of Prostate Cancer Does Darolutamide Treat?
Darolutamide is FDA-approved to treat several prostate cancer types, including:
- Non-metastatic castration-resistant prostate cancer (nmCRPC): This means the cancer is not yet spread to other parts of the body but continues to grow despite low testosterone levels.
- Metastatic hormone-sensitive prostate cancer (mHSPC): Prostate cancer that has spread but still responds to hormone therapy.
- Metastatic castration-sensitive prostate cancer (mCSPC): Advanced prostate cancer sensitive to hormone therapy and recently approved for treatment with Nubeqa.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Nubeqa was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
How Effective Is Darolutamide? What Do Clinical Trials Show?
The ARAMIS trial tested Darolutamide in 1,509 men with non-metastatic castration-resistant prostate cancer who had a PSA doubling time of 10 months or less (meaning their cancer was likely to progress quickly). Patients took Nubeqa (600 mg twice daily) plus standard hormone therapy or a placebo.
Key Results:
- Metastasis-Free Survival (time without spread):
- Darolutamide group: 40.4 months
- Placebo group: 18.4 months
This means Darolutamide nearly doubled the time patients lived without their cancer spreading.
Overall Survival: Darolutamide also improved overall survival compared to placebo.
Safety: Side effects were similar between the two groups, with fatigue being the most common. Serious side effects like seizures or fractures were rare and did not increase with Darolutamide. Treatment discontinuation rates were low.
ARASENS Trial (mHSPC with Docetaxel)
This trial studied 1,306 men with metastatic hormone-sensitive prostate cancer. Patients were treated with hormone therapy and chemotherapy (docetaxel) plus either Darolutamide or placebo.
Key Results:
- Risk of death: Reduced by 32.5% with Darolutamide (hazard ratio [HR] 0.68; P<0.001)
- Survival Benefits: Consistent across multiple secondary endpoints and patient subgroups.
- Disease status: 86.1% of patients had metastatic disease when first diagnosed.
- Safety: Serious side effects (grade 3 or 4) occurred in 66.1% of the Darolutamide group vs 63.5% of placebo group, with neutropenia (low white blood cells) being the most common. The side effects were manageable.
ARANOTE Trial (mCSPC)
In this study of 669 patients with metastatic castration-sensitive prostate cancer not receiving chemotherapy, Darolutamide was compared with placebo.
Key Results:
- Radiographic Progression-Free Survival (rPFS):
- Darolutamide group: median rPFS was not reached (meaning many patients did not have disease progression during the study period).
- Placebo group: median rPFS was 25 months.
- Hazard ratio: 0.54, indicating a 46% reduction in risk of progression or death with Darolutamide (P<0.0001).
- Overall Survival: Improvement was seen but did not reach statistical significance.
- Safety: Side effects consistent with previous trials; warnings for ischemic heart disease, seizures, and embryo-fetal toxicity apply.

Learn more about Prostate Cancer on OncoDaily.
How Is Darolutamide Given?
Nubeqa is administered by mouth as a tablet. The typical dose is 600 mg twice daily, which corresponds to two 300 mg tablets each time, taken with food. Patients should swallow tablets whole and avoid crushing or chewing them. When used in metastatic hormone-sensitive prostate cancer, Darolutamide is combined with chemotherapy (docetaxel), which is given as an intravenous infusion every three weeks for six cycles.
What Side Effects Can You Expect and How to Manage?
Common side effects reported with Nubeqa include fatigue or tiredness, pain in the arms or legs, skin rash, elevated liver enzymes detected by blood tests, and low white blood cell counts that increase infection risk.
Less common side effects may include chest pain or high blood pressure, irregular heartbeat, seizures (which are rare), urinary problems, bone fractures related to bone density changes, pneumonia, or other infections.
Managing these side effects involves regular blood tests to monitor liver function and blood counts. Rest and light exercise can help alleviate fatigue. Calcium, vitamin D, and weight-bearing exercise support bone health. Patients should promptly report symptoms such as fever, cough, chest pain, or urinary issues to their healthcare team. Blood pressure and heart symptoms should also be monitored and treated as needed.

What Should You Avoid While Taking Darolutamide?
Patients should avoid starting new medications without discussing them with their doctor, as Nubeqa can interact with drugs like topotecan, glyburide, and chlorothiazide. Herbal supplements that affect liver enzymes should also be avoided. Alcohol consumption and driving should be approached cautiously until patients understand how Nubeqa affects them. It is important to inform the healthcare provider of all medicines and supplements currently taken.
What Can You Expect During Treatment?
Patients take Darolutamide tablets twice daily with food until advised to stop by their doctor. When combined with chemotherapy, intravenous docetaxel is administered every three weeks for six cycles. Treatment monitoring includes regular doctor visits and blood tests to track response and side effects. Side effects are managed proactively to maintain quality of life throughout treatment.
What Is the Long-Term Outlook With Darolutamide?
Darolutamide is not a cure, but it can help control prostate cancer for a long time, delaying spread and improving survival. Many patients stay on treatment for months or years with a good quality of life. If cancer progresses, your doctor may suggest other treatments. Ongoing research aims to find the best ways to combine Nubeqa with new therapies for even better long-term outcomes.
Real-Life Effectiveness
Clinical trials have shown darolutamide’s benefits, and real-world studies like SOGUG-PRINCIS are confirming its effectiveness in everyday practice. Patient feedback generally highlights good tolerance and improved cancer control.
Looking Ahead: The Future of Darolutamide Treatment
Scientists continue to study darolutamide for new uses, including combinations with immunotherapy or targeted drugs. This research could help even more patients with prostate and possibly other cancers. Nubeqa offers a promising option for men with advanced prostate cancer, helping slow disease progression with manageable side effects. If you or a loved one are considering Nubeqa, talk to your healthcare provider to understand if it’s right for you and how to best manage your treatment journey.
Ongoing Research and Future Directions
Ongoing studies such as the SOGUG-PRINCIS trial collect real-world data on Darolutamide’s effectiveness outside clinical trials. Researchers are also exploring its use in combination with other therapies and in different prostate cancer types. There is continued investigation into whether Nubeqa may benefit other cancers or conditions involving androgen receptor signaling.
If you’re a healthcare provider, access the professional version here.