Darolutamide is a medication used in the treatment of prostate cancer. It belongs to a class of drugs known as androgen receptor inhibitors, which block the effects of male hormones (androgens) that can promote the growth of prostate cancer cells. With its targeted mechanism of action and generally well-tolerated safety profile, darolutamide has become an important option for certain patients with advanced prostate cancer.
This article will explore how darolutamide works, its approved uses, potential side effects, recommended dosage, what to expect during treatment.
Which company produced Darolutamide?
Darolutamide, marketed under the brand name Nubeqa, was developed through a strategic partnership between Orion Corporation and Bayer AG. Orion, a Finnish pharmaceutical company with expertise in oncology and drug manufacturing, originally discovered and patented the molecule (ODM‑201/BAY‑1841788) around 2010–2011. Recognizing the global potential of this treatment for prostate cancer, Orion joined forces with Bayer—a German multinational with extensive experience in drug development and commercialization.
While Orion continues to manufacture the active pharmaceutical ingredient (API) and final drug product through its subsidiary Fermion, Bayer took on responsibility for late-stage clinical development, global regulatory filings, and worldwide commercialization. Orion co-promotes Nubeqa in Europe and receives royalties and milestone payments from Bayer, who holds the global commercial rights. This collaboration effectively combines Orion’s innovation and manufacturing strength with Bayer’s global infrastructure to deliver darolutamide to patients worldwide.
How does Darolutamide work?
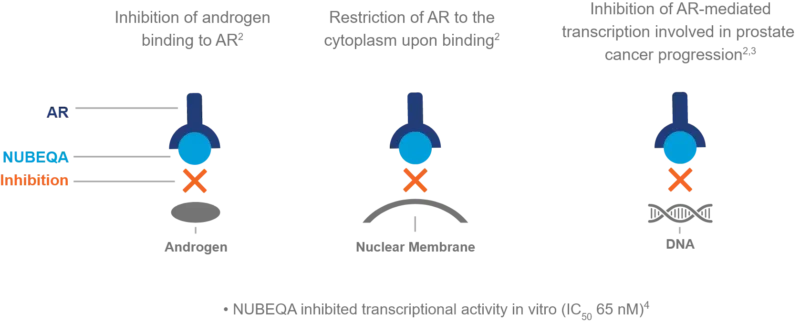
Darolutamide is a nonsteroidal antiandrogen used to treat certain types of prostate cancer by blocking the effects of male hormones (androgens), such as testosterone, that fuel cancer growth. It works by competitively binding to androgen receptors on prostate cancer cells, preventing androgens from activating these receptors. This blocks the receptor’s movement into the cell nucleus and the activation of genes that promote tumor growth.
The drug also produces an active metabolite, keto-darolutamide, which contributes to its therapeutic effect. Notably, darolutamide has limited penetration across the blood-brain barrier, which may reduce the risk of central nervous system side effects compared to some other antiandrogens. By disrupting androgen receptor signaling, Nubeqa helps slow the progression of prostate cancer, especially in cases that no longer respond to standard hormone therapy.
What Cancers Is Darolutamide Approved to Treat?
Nubeqa has received multiple FDA approvals for the treatment of prostate cancer. On July 30, 2019, the U.S. Food and Drug Administration (FDA) approved Nubeqa for non-metastatic castration-resistant prostate cancer (nmCRPC), providing a new treatment option for men whose cancer progresses despite low testosterone levels but has not yet spread.
Later, on August 5, 2022, the FDA approved a supplemental New Drug Application (sNDA) for darolutamide in combination with docetaxel for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC). Most recently, on June 3, 2025, the FDA granted approval for Nubeqa for the treatment of metastatic castration-sensitive prostate cancer (mCSPC), further expanding its role across multiple stages of advanced prostate cancer. These approvals highlight Nubeqa’s growing importance in the prostate cancer treatment landscape.

Learn more about Prostate Cancer on OncoDaily.
What research is behind the approval?
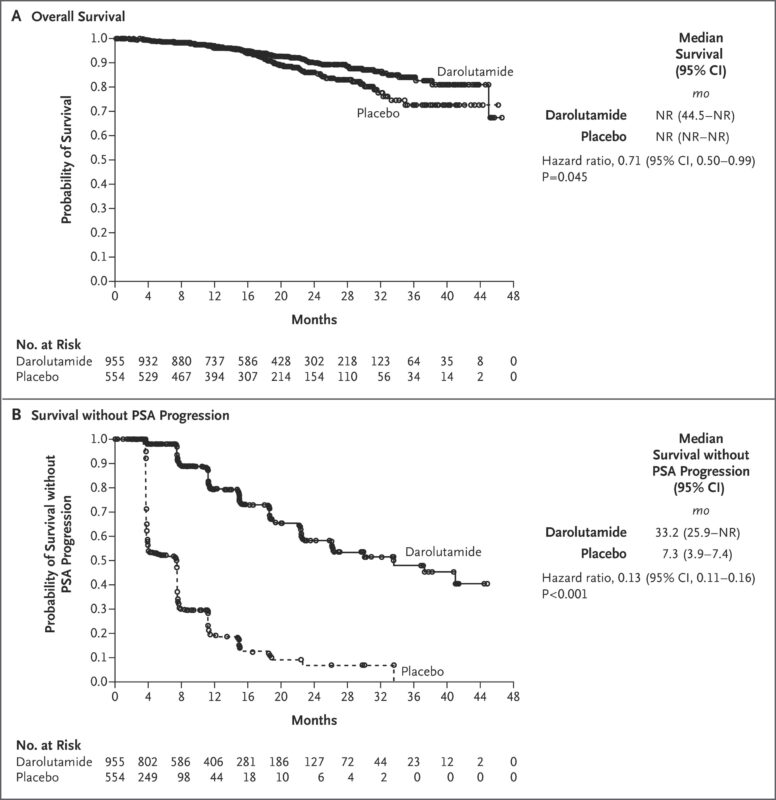
The FDA approved Nubeqa for treating non-metastatic castration-resistant prostate cancer (nmCRPC) based on the ARAMIS phase 3 trial, published in the New England Journal of Medicine in 2019.
This randomized, placebo-controlled trial included 1,509 men with nmCRPC and a PSA doubling time of 10 months or less. Patients received darolutamide (600 mg twice daily) plus androgen deprivation therapy. Darolutamide significantly extended metastasis-free survival to 40.4 months, compared to 18.4 months with placebo (HR 0.41; P<0.001), and improved overall survival and other key outcomes.
Side effects were similar between groups, with fatigue being the most common. Serious adverse events like seizures or fractures were not increased, and treatment discontinuation rates were low and comparable. The ARAMIS trial confirmed Nubeqa as a safe and effective treatment for nmCRPC.
On June 3, 2025, the FDA approved darolutamide for metastatic castration-sensitive prostate cancer (mCSPC). The approval was based on the ARANOTE trial, a randomized, double-blind study of 669 patients, which showed darolutamide significantly improved radiographic progression-free survival (rPFS) compared to placebo (median rPFS not reached vs. 25 months; HR 0.54; p < 0.0001), though overall survival improvement was not statistically significant.
Adverse effects were consistent with known darolutamide safety, including warnings for ischemic heart disease, seizures, and embryo-fetal toxicity. The recommended dose is 600 mg orally twice daily with food until disease progression or unacceptable toxicity.
Darolutamide Combinations and Treatment Outcomes
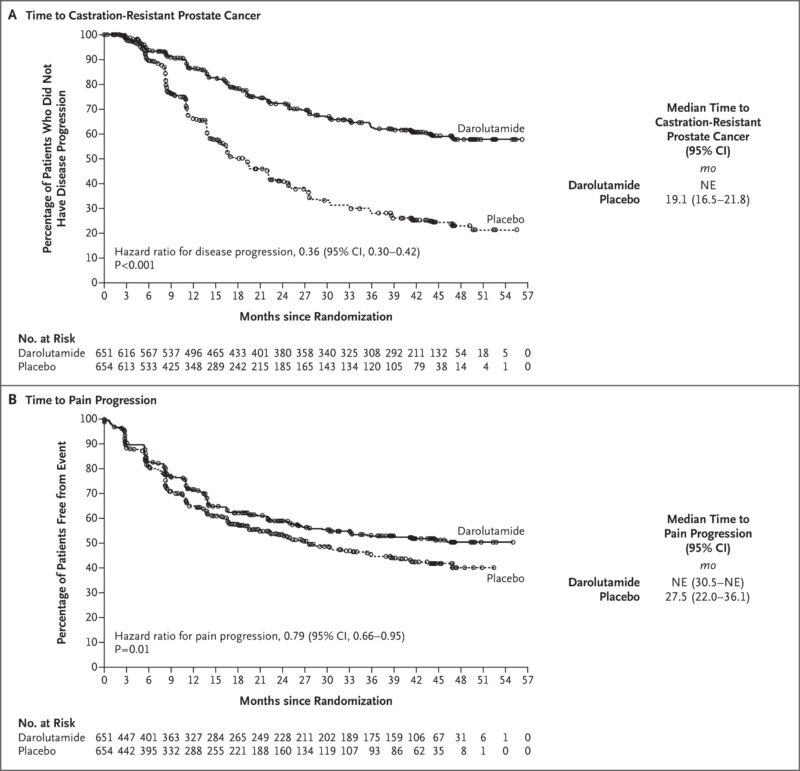
The use of Nubeqa in combination with docetaxel for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC) is based on the pivotal ARASENS phase 3 trial. On August 5, 2022, the FDA approved this combination following strong clinical evidence showing a significant survival benefit.
The trial, published in the New England Journal of Medicine on February 17, 2022, enrolled 1,306 patients who received either darolutamide or placebo, both alongside androgen deprivation therapy (ADT) and docetaxel. The results showed that darolutamide reduced the risk of death by 32.5% compared to placebo (HR 0.68; 95% CI, 0.57–0.80; P<0.001). Survival benefits were consistent across secondary endpoints and subgroups. Notably, 86.1% of patients had metastatic disease at initial diagnosis.
The safety profile was manageable, with grade 3 or 4 adverse events seen in 66.1% of patients in the darolutamide arm and 63.5% in the placebo group. The most common severe side effect was neutropenia. These findings support darolutamide plus ADT and docetaxel as an effective new standard for patients with mHSPC.
Darolutamide side effects and its management
Darolutamide is an androgen receptor inhibitor approved for the treatment of prostate cancer. It is generally well tolerated, but like all medications, it may cause side effects. Understanding what to expect and how to manage potential issues is essential for maintaining quality of life during treatment.
Common Side Effects
The most commonly reported side effects of Nubeqa include fatigue, pain in the limbs, and skin rash. Some patients may also experience elevated liver enzymes, which can be detected through routine blood tests. A reduction in white blood cell count has been observed, which may increase the risk of infections. These side effects are usually mild to moderate in intensity and manageable with supportive care or monitoring.
Less Common Side Effects
Less frequently, patients may experience cardiovascular symptoms such as chest pain, high blood pressure, or irregular heartbeat. Seizures have been rarely reported. Some individuals may notice urinary problems or discomfort during urination. A small percentage of patients may experience bone fractures due to changes in bone density, and infections like pneumonia have also been observed. While these events are not common, it’s important to promptly report any unusual symptoms to your healthcare provider.
Management of Side Effects
Managing Nubeqa side effects starts with regular medical monitoring, including blood tests to assess liver function and blood counts. Fatigue may be addressed through lifestyle modifications, such as incorporating rest periods and light physical activity. Bone health can be supported with calcium and vitamin D supplementation, as well as weight-bearing exercise if appropriate. Patients should be vigilant for signs of infection, such as fever or persistent cough, and report these symptoms immediately. If cardiovascular symptoms occur, blood pressure and heart health should be evaluated and managed as needed. In all cases, working closely with your oncology team ensures timely intervention and helps maintain the effectiveness of treatment.

What is the Recommended Dosage of Darolutamide?
Darolutamide comes as a 300 mg oral tablet. The standard dose is 600 mg twice daily, taken with food. For nonmetastatic castration-resistant prostate cancer (nmCRPC), treatment continues until progression or unacceptable side effects. Patients should also receive a GnRH analog or have had a bilateral orchiectomy.
For metastatic hormone-sensitive prostate cancer (mHSPC), darolutamide is used with docetaxel (75 mg/m² IV every 3 weeks for 6 cycles). Start docetaxel within 6 weeks of beginning darolutamide. If severe side effects occur, the dose can be reduced to 300 mg twice daily, then increased again once symptoms improve. Minimum dose is 300 mg BID.
How is Darolutamide administered?
Darolutamide should be taken by mouth with food. Each tablet must be swallowed whole—do not crush, chew, or split. If a dose is missed, it can be taken as soon as remembered, as long as it’s before the next scheduled dose. Do not take two doses at once to make up for a missed one.
Store the medication at room temperature 20°C to 25°C. Short-term temperature changes between 15°C to 30°C are acceptable. Always keep the bottle tightly closed to protect the tablets from moisture and contamination.
What to Avoid During Darolutamide Treatment?
Patients receiving darolutamide should avoid taking other medications without consulting their healthcare provider. Darolutamide may increase blood levels of certain drugs such as topotecan, glyburide, and chlorothiazide, potentially raising the risk of side effects. Its effectiveness may also be altered by strong CYP3A4 or P-glycoprotein (P-gp) inhibitors or inducers.
It should be taken with food to ensure proper absorption. Herbal supplements that affect liver enzymes should be avoided, as they may interfere with how Nubeqa works. Although darolutamide is not commonly associated with drowsiness, caution is advised when driving or consuming alcohol until individual tolerance is known.
Patients should inform their healthcare provider of all prescription drugs, over-the-counter medications, and supplements they are taking to minimize the risk of interactions.
Darolutamide effectiveness over time
Nubeqa has shown sustained effectiveness in treating prostate cancer across multiple clinical trials. In the ARAMIS trial, it significantly prolonged metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer, nearly doubling the time without disease spread compared to placebo. The ARASENS trial further demonstrated that darolutamide, when combined with docetaxel and androgen-deprivation therapy, reduced the risk of death by 32.5% in patients with metastatic hormone-sensitive prostate cancer.
More recently, the ARANOTE trial confirmed that Nubeqa plus androgen-deprivation therapy significantly delayed radiological progression in metastatic hormone-sensitive prostate cancer patients who were not receiving chemotherapy. These consistent results highlight darolutamide’s long-term benefits in delaying disease progression and improving overall survival, all while maintaining a favorable safety profile over time.
Ongoing trials with Darolutamide
The SOGUG-PRINCIS study is a national, multicenter observational project evaluating the real-world effectiveness of newly funded drugs for genitourinary cancers in Spain. It collects data retrospectively and prospectively without altering patient treatment decisions. The study acts as a registry, adding subprojects for each new drug to validate clinical trial results with real-world evidence. Current drugs studied include darolutamide, nivolumab, and enfortumab vedotin.
FAQ
How does Darolutamide work in prostate cancer treatment?
It is an androgen receptor inhibitor that blocks signals that promote prostate cancer cell growth, helping to slow disease progression.
Who manufactures Nubeqa?
Darolutamide is produced by Bayer and Orion Pharma.
What is the recommended dosage of Darolutamide?
The standard dosage is 600 mg taken orally twice daily with food, continued until disease progression or unacceptable side effects.
What are the most common side effects of Darolutamide?
Common side effects include fatigue, nausea, and rash, though it generally has a favorable safety profile compared to other androgen receptor inhibitors.
How should Darolutamide be administered?
Nubeqa tablets should be swallowed whole with food, taken twice daily at the same times each day.
What should patients avoid while taking Darolutamide?
Patients should avoid strong CYP3A4 inhibitors or inducers and consult their doctor about potential drug interactions.


