Bemarituzumab is a first-in-class monoclonal antibody designed to target fibroblast growth factor receptor 2b (FGFR2b), a biomarker identified in a subset of gastric and gastroesophageal junction (GEJ) adenocarcinomas. Clinical development has focused on biomarker-selected, HER2-negative advanced disease, particularly in the first-line setting in combination with chemotherapy. The phase 2 FIGHT trial provides the most mature data currently available for this agent (Wainberg et al., 2024).
This clinician-focused overview summarizes the mechanism of action, clinical uses, dosing strategy, efficacy expectations, and toxicity profile based on published trial data.

Read About Gastric Cancer on OncoDaily
What Is Bemarituzumab?
Bemarituzumab is a humanized monoclonal antibody selective for FGFR2b, the IIIb splice isoform of FGFR2. The FGF/FGFR pathway plays a central role in tumor cell proliferation, survival, and progression. FGFR2b overexpression has been observed in approximately 30% of HER2-negative gastric cancers and has been associated with poorer clinical outcomes (Wainberg et al., 2024).
Bemarituzumab acts through two mechanisms. First, it blocks ligand binding to FGFR2b, inhibiting downstream signaling pathways that drive tumor growth. Second, due to its afucosylated Fc structure, it enhances binding to FcγRIIIa/CD16a on natural killer cells, promoting antibody-dependent cellular cytotoxicity against FGFR2b-expressing tumor cells (Wainberg et al., 2024).
This dual mechanism distinguishes bemarituzumab from small-molecule FGFR inhibitors and supports its development in biomarker-selected populations.
Uses in Cancer
Bemarituzumab has been evaluated in advanced gastric and GEJ adenocarcinoma in the phase 1/2 FIGHT trial (NCT03694522). Eligible patients had HER2-negative, unresectable locally advanced or metastatic disease with FGFR2b overexpression determined by immunohistochemistry and/or FGFR2 gene amplification detected by circulating tumor DNA.
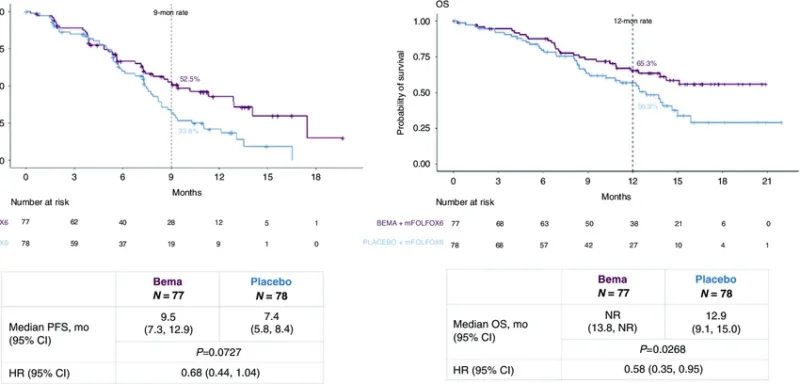
In the randomized phase 2 portion, bemarituzumab was combined with modified FOLFOX6 (mFOLFOX6) chemotherapy and compared with placebo plus mFOLFOX6 as first-line treatment.
The final analysis after a minimum follow-up of 24 months demonstrated numerically longer progression-free survival and overall survival with bemarituzumab plus chemotherapy compared with chemotherapy alone (Wainberg et al., 2024).
In the overall intention-to-treat population:
- Median progression-free survival was 9.5 months with bemarituzumab-mFOLFOX6 versus 7.4 months with placebo-mFOLFOX6 (HR 0.72; 95% CI 0.49–1.08).
- Median overall survival was 19.2 months versus 13.5 months (HR 0.77; 95% CI 0.52–1.14).
- Objective response rate was 48.1% versus 33.3%.
The most pronounced benefit was observed in patients with FGFR2b overexpression in at least 10% of tumor cells (2+/3+ staining by immunohistochemistry). In this subgroup:
- Median progression-free survival was 14.0 months versus 7.3 months (HR 0.43; 95% CI 0.26–0.73).
- Median overall survival was 24.7 months versus 11.1 months (HR 0.52; 95% CI 0.31–0.85).
- The 24-month overall survival rate was 51.3% versus 21.3%.
These findings support the clinical relevance of biomarker enrichment when selecting patients for FGFR2b-targeted therapy. Ongoing phase 3 trials (NCT05052801, NCT05111626) are evaluating bemarituzumab in this setting to confirm these observations.
Dosage and Administration
In the phase 2 FIGHT trial, bemarituzumab was administered intravenously at a dose of 15 mg/kg every two weeks, with an additional 7.5 mg/kg dose on cycle 1 day 8.
All patients received standard mFOLFOX6 chemotherapy every two weeks, consisting of oxaliplatin, leucovorin, and 5-fluorouracil. Treatment continued until disease progression or unacceptable toxicity.
Dose adjustments for chemotherapy were performed per protocol. Bemarituzumab discontinuation occurred in cases of treatment-emergent adverse events that did not resolve according to predefined criteria.
Side Effects and Safety Profile
Nearly all patients in both arms of the FIGHT trial experienced at least one treatment-emergent adverse event. Grade 3 or higher adverse events were reported in 82.9% of patients receiving bemarituzumab-mFOLFOX6 and 75.3% receiving placebo-mFOLFOX6 (Wainberg et al., 2024). The most clinically distinctive toxicity associated with bemarituzumab was ocular toxicity, particularly corneal adverse events.
Any-grade corneal adverse events occurred in 67.1% of patients treated with bemarituzumab compared with 10.4% in the placebo arm. Grade 3 corneal adverse events occurred in 27.6% of patients receiving bemarituzumab. Importantly, no grade 4 or serious corneal events were reported.
Corneal events led to treatment discontinuation in 31.6% of patients in the bemarituzumab arm. Median time to onset of any-grade corneal events was 16.9 weeks.
Other commonly reported adverse events included nausea, decreased neutrophil count, diarrhea, anemia, decreased appetite, constipation, vomiting, and elevations in liver enzymes. Serious adverse events occurred at similar rates between treatment arms. Fatal treatment-emergent adverse events were reported in 6.6% of patients in the bemarituzumab arm and 5.2% in the placebo arm.
No new safety signals emerged with longer follow-up in the final analysis. The ocular toxicity profile has informed modifications in ongoing phase 3 trials, including strategies for earlier detection and mitigation.

Read About Gastric Cancer Remission Rate on OncoDaily
What Should Clinicians and Patients Expect?
For clinicians, bemarituzumab represents a biomarker-driven therapeutic strategy in HER2-negative advanced gastric cancer. The data suggest that patients with higher levels of FGFR2b expression derive greater benefit, emphasizing the importance of precise biomarker testing.
For patients with FGFR2b overexpression in at least 10% of tumor cells, the observed median overall survival exceeding 24 months in the phase 2 trial is notable in the context of historically limited outcomes in advanced gastric cancer.
However, expectations must also include close monitoring for ocular adverse events, particularly corneal toxicity. Early recognition and management are critical, as many events resolved or improved over time.
The phase 2 FIGHT trial was not powered for formal hypothesis testing, and confirmatory phase 3 data are required before establishing a new standard of care. Nonetheless, the magnitude and consistency of benefit in the biomarker-enriched subgroup support continued clinical development.
Conclusion
Bemarituzumab is an investigational FGFR2b-targeted monoclonal antibody evaluated in first-line HER2-negative advanced gastric and GEJ adenocarcinoma. In the randomized phase 2 FIGHT trial, bemarituzumab combined with mFOLFOX6 demonstrated numerically improved progression-free and overall survival, with the most pronounced benefit in patients with FGFR2b overexpression in at least 10% of tumor cells.
The safety profile was characterized by manageable but frequent corneal adverse events. Ongoing phase 3 studies are designed to confirm efficacy and refine toxicity management.
As biomarker-guided therapy continues to reshape gastric cancer treatment, FGFR2b targeting with bemarituzumab may represent a meaningful addition to precision oncology strategies pending confirmatory results.


