Antibody-drug conjugates (ADCs) are a class of targeted cancer therapies that combine monoclonal antibodies with cytotoxic drugs, allowing for precise delivery of the drug to cancer cells. While ADCs have shown significant promise in treating various cancers, they are not without side effects. One of the notable adverse effects is ocular toxicity, which can manifest as vision changes, retinal damage, or other eye-related issues. This toxicity is particularly concerning as it may limit the therapeutic use of certain ADCs, necessitating close monitoring and management during treatment.
The concept of antibody-drug conjugates (ADCs) was first introduced in 1900 by German Nobel laureate Paul Ehrlich. He envisioned drugs that could selectively target tumor cells while sparing healthy ones, calling them a “magic bullet” for their precision. While most ADCs developed to date target cancer, they hold significant potential for treating other diseases. Currently, the U.S. Food and Drug Administration (FDA) has approved ten ADCs, with over 90 more in clinical development worldwide. Over the past 30 years, monoclonal antibodies have transitioned from research tools to powerful therapeutic agents
A highly potent cytotoxic drug is designed to kill target cells after being taken up by tumor cells, though it can have significant systemic toxicity. It is attached to a stable linker that stays intact in the bloodstream but releases the drug inside tumors. Monoclonal antibodies are linked to these drugs to target cancer cells precisely while reducing harm to healthy cells. This method boosts the effectiveness of the antibodies, increases tumor selectivity, improves drug tolerability, and lowers overall exposure compared to traditional chemotherapy or biologics.
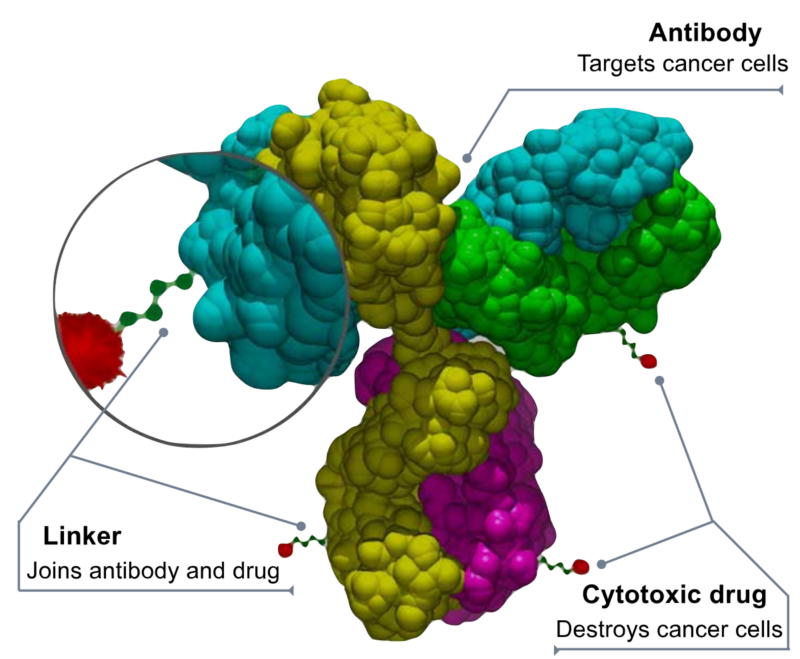
How Do Immunotoxins Kill Cancer Cells?
Antitumor activity is achieved by attaching antibodies to molecules that cause cell death after binding and being taken up by the cell. These molecules can be toxic agents, bacterial or plant toxins (immunotoxins), or radiopharmaceuticals.Immunotoxins are engineered proteins that combine an antibody or antibody fragment targeting a tumor with a toxin, like diphtheria toxin or pseudomonas exotoxin A. The only FDA-approved immunotoxin currently is denileukin diftitox, used to treat CD25-positive cutaneous T-cell lymphoma.
Another immunotoxin, moxetumomab pasudotox, targets CD22 and has shown strong effectiveness in treating hairy cell leukemia. It is currently being tested in a multicenter trial for relapsed or refractory cases.Immunotoxins have been less effective in solid tumors due to immune responses reducing their activity. However, the anti-mesothelin immunotoxin SS1P caused significant tumor reduction in treatment-resistant mesothelioma when combined with pentostatin and cyclophosphamide.
Gemtuzumab ozogamicin was approved by the FDA in 2000 for acute myeloid leukemia but was withdrawn in 2010 after it didn’t meet effectiveness targets in clinical trials. Since then, two other antibody-drug conjugates have been approved: trastuzumab emtansine in 2013 for metastatic breast cancer, and brentuximab vedotin in 2011 for refractory Hodgkin’s lymphoma and systemic anaplastic large-cell lymphoma.
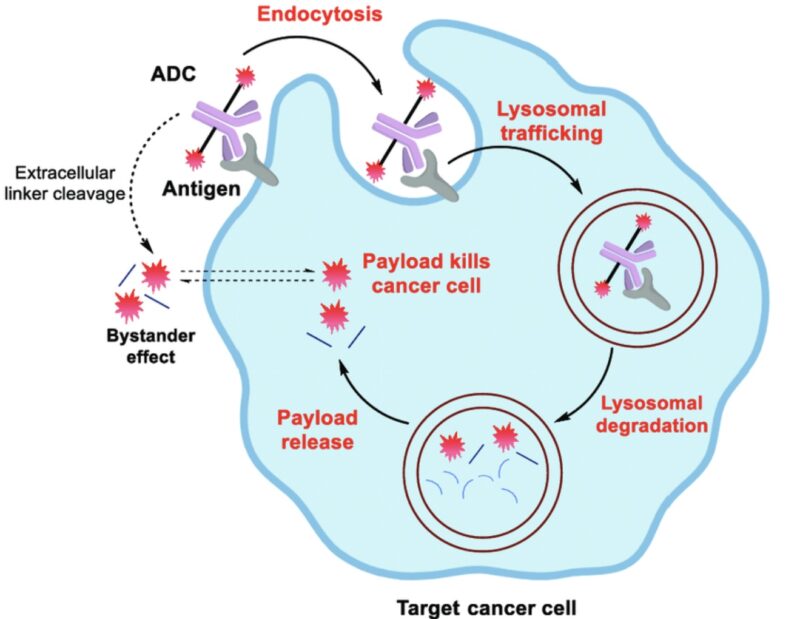
What Are the Common Types of Diseases Treated with ADCs?
More than 40 antibody–drug conjugates are in clinical development for various haematological malignant diseases and solid tumours.
Currently, antibody-drug conjugates (ADCs) are approved for the treatment of various cancers. These include lymphoma, such as diffuse large B-cell lymphoma (the most common type of non-Hodgkin lymphoma), relapsed Hodgkin lymphoma, systemic anaplastic large-cell lymphoma, and certain cases of stage 3 or 4 classical Hodgkin lymphoma. They are also used for specific cases of leukemia, including B-cell precursor acute lymphoblastic leukemia, relapsed or refractory hairy cell leukemia, and acute myeloid leukemia in select adults and children. ADCs are approved for relapsed or refractory multiple myeloma, as well as metastatic triple-negative and HER2-positive breast cancer. They are also used in the treatment of early-stage post-surgical breast cancer and advanced breast cancer previously treated with trastuzumab and chemotherapy.
Additionally, ADCs are used for urothelial carcinoma, the most common type of bladder cancer, especially when it worsens after chemotherapy and immunotherapy. Finally, they are approved for recurrent or metastatic cervical cancer following chemotherapy.
How Effective Are ADCs in Cancer Treatment?
The effectiveness of ADCs in cancer treatment depends on various factors, including the specific drug, tumor type, and patient characteristics. Clinical trials and real-world studies have demonstrated promising outcomes, with ADCs improving response rates and survival in select populations. However, their use also comes with unique safety considerations and potential resistance mechanisms.
ADCs in Advanced HER2-Positive Breast Cancer.
With the improvement in the overall survival (OS) of breast cancer, drug resistance has become a great challenge. ADCs are among the most promising drugs for treating tumors. The approval of trastuzumab deruxtecan (DS-8201a) and trastuzumab emtansine (T-DM1) improved the prognosis of HER2-positive breast cancer.ADCs revised the treatment guidelines for HER2-positive breast cancer and provided patients with promising treatment options. Compared with capecitabine plus lapatinib, T-DM1 significantly improved the median progression-free survival (PFS) (6.4 months vs. 9.6 months), and median OS (25.1 months vs. 30.9 months) in the phase III EMILIA study.[Verma S, N Engl J Med 2012]
Based on the results of the EMILIA study, T-DM1 was approved by the Food and Drug Administration in 2013 for the treatment of patients with HER2-positive metastatic breast cancer (mBC) who previously received trastuzumab and taxane, separately or in combination.[Amiri-Kordestani L, Clin Cancer Res 2014]
TH3RESA further confirmed the efficacy and safety of T-DM1 in HER2-positive advanced breast cancer.[Krop IE, Lancet Oncol 2017]
What Are the Most Common Side Effects of ADCs?
Antibody-drug conjugates (ADCs) are associated with various toxicities, including hematologic side effects such as neuropathy, thrombocytopenia, febrile neutropenia, lymphopenia, and anemia. Common all-grade adverse events include lymphopenia, nausea, neutropenia, blurred vision, and peripheral neuropathy, while severe (Grade 3/4) toxicities primarily involve neutropenia, thrombocytopenia, febrile neutropenia, and lymphopenia. Payload-specific toxicities vary: MMAE ADCs often cause anemia, neutropenia, and peripheral neuropathy; DM1 ADCs are linked to thrombocytopenia and hepatic toxicity; MMAF and DM4 ADCs frequently result in severe ocular toxicity. PBD-ADCs exhibit a consistent toxicity profile, including vascular leak syndrome, bone marrow suppression, liver enzyme elevation, gastrointestinal issues, metabolic effects, neuropathy, musculoskeletal pain, dyspnea, fatigue, and kidney injury. [Saber H., Regul. Toxicol. Pharm. 2019]
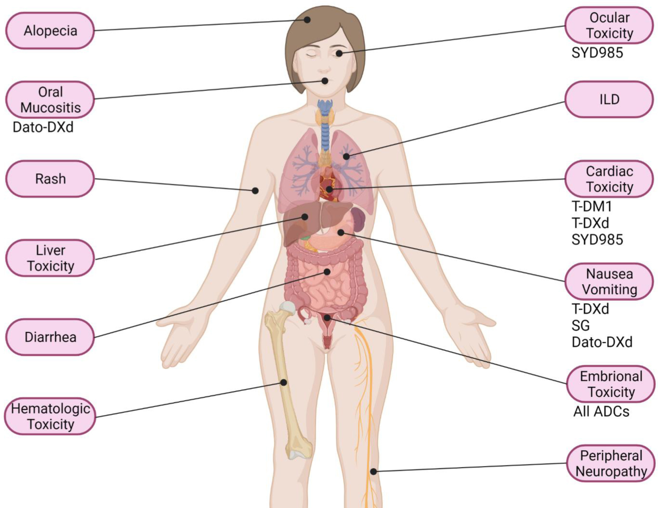
Andrea D’Arienzo elClinicalMedicine 2023 Main AEs described with ADCs for BC treatment.
Drugs needing prophylaxis use or medical/laboratory screening are indicated.
Ocular Toxicity Of ADCs.
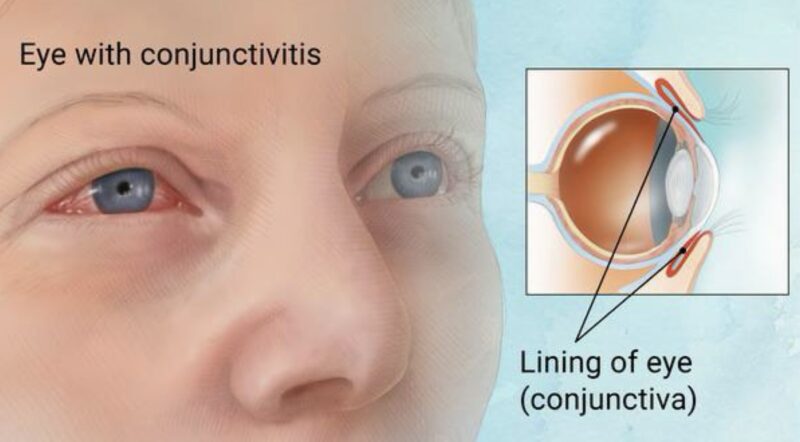
Ocular toxicities are common with antibody-drug conjugates (ADCs), especially those used for solid tumors. These toxicities can range from mild irritation to severe vision problems. Common side effects include keratitis, dry eye, corneal microcysts, and conjunctivitis. Blurred vision is the most frequent symptom, but some patients may also experience decreased visual acuity or double vision. Rare but serious issues like optic neuropathy and cortical blindness may occur, with the latter often linked to reversible posterior leukoencephalopathy syndrome (RPLS).
The severity of these ocular issues can vary. Some patients have mild symptoms, while others may develop more significant vision problems that could require therapy adjustments or discontinuation. ADCs targeting solid tumors, like tisotumab vedotin, are more commonly associated with ocular AEs compared to those targeting hematologic malignancies. However, other ADCs such as polatuzumab vedotin, enfortumab vedotin, and trastuzumab emtansine also carry a risk for ocular toxicities like dry eye and blurred vision.
Management of these ocular AEs is important for patient comfort and safety, as they can impact quality of life.
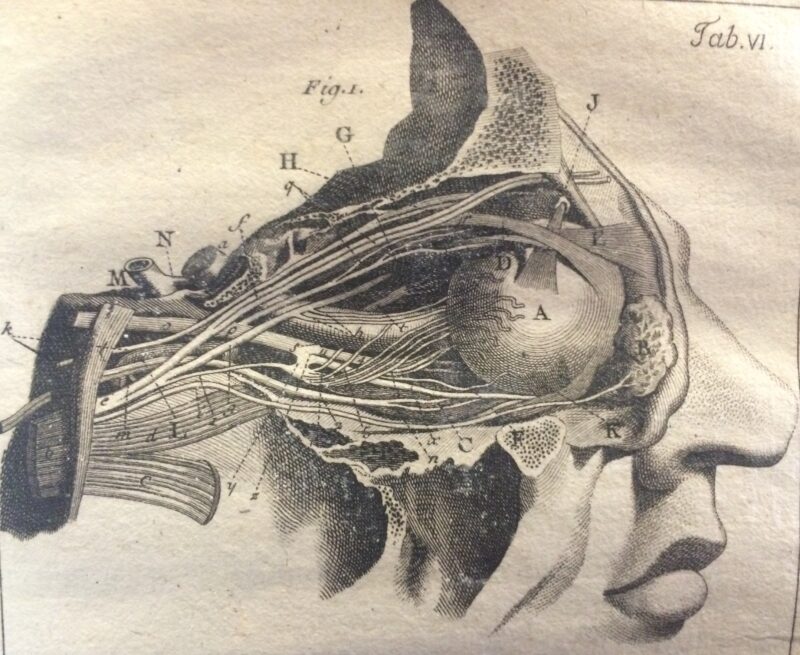
Structure of the eye and optic nerves from Degravers (1780).
Mechanism Of Ocular Toxicity in ADCs.
ADC-induced ocular toxicity can be caused by both off-target and on-target mechanisms.
Off-target toxicity typically results from the cytotoxic payload, while on-target toxicity occurs when the drug binds to its intended receptor.[Jaffry M, J Ocul Pharmacol Ther]Ocular toxicity has been reported with maytansinoid- and monomethyl auristatin F (MMAF)-containing ADCs, even when the targeted antigens (like CD19, folate receptor alpha, and mesothelin) are not expressed in ocular tissues. Ocular toxicity risks varied among MMAE-containing ADCs, with higher risks seen in tisotumab vedotin (targeting TF) and enfortumab vedotin (targeting Nectin-4). [de Goeij BE, Curr Opin Immunol. 2016]
HER2-targeting ADCs also showed increased ocular toxicity. This suggests that ocular toxicity can be influenced by both the cytotoxic payload and the targeting antibodies. Preclinical studies have shown that TF is expressed in the human conjunctiva and is linked to conditions like pterygium and age-related macular degeneration. Additionally, proteins like HER2 and Nectin-4, which are present in conjunctival and corneal cells, are involved in important signaling pathways like ERK and PI3K-AKT, which are crucial for maintaining ocular health.[Lui Z, Cornea 2001]
Diagnostic Methods for ADC-Induced Ocular Toxicity
The diagnosis of ocular toxicity from ADCs involves clinical assessments, imaging, and functional tests. Patient history and symptom assessment focus on blurred vision, photophobia, dry eye, and ocular discomfort, which often develop within weeks of ADC therapy. Slit-lamp biomicroscopy is essential for detecting microcystic epithelial changes, corneal haze, or punctate keratopathy, common in ADC-related toxicity (e.g., mirvetuximab soravtansine).
Fluorescein and lissamine green staining identify corneal and conjunctival epithelial damage, while corneal confocal microscopy (CCM) provides high-resolution imaging of epithelial microcysts and nerve alterations. Tear film assessments, including Schirmer’s test and tear breakup time (TBUT), help evaluate ADC-induced dry eye. Optical coherence tomography (OCT) can detect corneal and retinal structural changes. Regular visual acuity testing and contrast sensitivity assessments track functional impairment. Early diagnosis enables dose adjustments, temporary discontinuation, or supportive interventions to manage ADC-induced ocular toxicity.
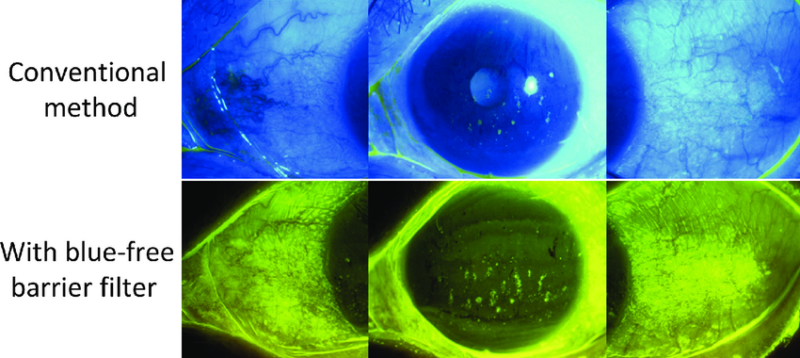
The use of a yellow filter allows assessment of conjunctival staining with fluorescein (Indian Journal of Ophthalmology)
Prevention Strategies for ADC-Related Toxicity
Optimizing the design and clinical management of antibody-drug conjugates (ADCs) is essential for balancing efficacy and safety. Careful selection of the target, antibody, cytotoxic payload, and linker plays a critical role in determining an ADC’s therapeutic potential. Modifications to the antibody, linker, or payload are being explored to improve tolerability. The linker must be stable enough to prevent premature drug release in circulation while ensuring efficient payload release upon internalization into the target cell.
In clinical trials, optimizing the dose and treatment schedule can enhance ADC safety. Strategies such as dose-capping, fractionated administration, and limiting treatment duration help reduce toxicity. Supportive care measures, including G-CSF prophylaxis for patients at high risk of febrile neutropenia and prophylactic antibiotics for those with low neutrophil counts, can further mitigate adverse effects.
Innovative approaches like the Indirect Active Targeting (INTACT) strategy—which uses prebinding antibodies with cell membrane vesicles to reduce off-target interactions—offer potential solutions for improving ADC selectivity. Additionally, a deeper understanding of unconventional toxicity mechanisms can help refine diagnostic and management practices.
Finally, identifying predictive biomarkers for ADC-related toxicities may allow for better patient selection and risk stratification, ultimately improving safety and treatment outcomes. By integrating these strategies, ADC development can continue evolving toward more effective and tolerable therapies.
You Can Also Read Immunotherapy for Lymphoma by OncoDaily
Can ADCs Be Used with Radiotherapy?
Combining antibody-drug conjugates (ADCs) with radiotherapy enhances cancer treatment by improving specificity, efficacy, and personalization. ADCs target cancer cells while minimizing damage to healthy tissue, reducing off-target effects. This combination can more effectively inhibit tumor growth and improve survival rates. ADCs also help personalize radiotherapy by selectively sensitizing certain cancer cells, enhancing tumor control and reducing side effects. By delivering radiosensitizing drugs directly to tumors, ADCs improve radiotherapy’s effectiveness while minimizing systemic toxicity. ADCs can overcome drug resistance and be combined with other therapies, such as immunotherapy or DNA damage response inhibitors, for a more comprehensive treatment approach.
Combining antibody-drug conjugates (ADCs) with radiotherapy shows promise in treating various cancers. In non-small cell lung cancer (NSCLC), targeting radiation-inducible TIP1 with a radiosensitizing ADC enhances radiotherapy efficacy.Han M, Cancer Lett. 2013
For advanced bladder cancer, ADCs like enfortumab vedotin and sacituzumab govitecan are approved in the US and can be used alongside radiation. Jonasch E, Ann Oncol
ADCs have also proven effective in HER2-positive cancers, including breast, stomach, lung, gynecologic, and bladder cancers, with trastuzumab emtansine approved in 2013 for metastatic breast cancer treatment.
You can also watch Brad McGregor on Dual Antibody-Drug Conjugates for Bladder Cancer | Chandler Park by OncoDaily
Written by Margarita Pozlikyan MD
FAQ
What are the common ocular adverse events (AEs) associated with antibody-drug conjugates (ADCs)?
Common ocular AEs include dry eye, blurred vision, keratopathy, and conjunctivitis. Enfortumab vedotin and tisotumab vedotin are particularly noted for high incidence rates of these AEs, with dry eye symptoms affecting up to 36% of patients.
Which ADCs are most frequently linked to ocular toxicity?
The ADCs most frequently associated with ocular toxicity include enfortumab vedotin, tisotumab vedotin, trastuzumab emtansine, and belantamab mafodotin. Tisotumab vedotin shows the strongest signals for ocular toxicity.
What is the typical onset time for ocular AEs after ADC administration?
The median time to onset (TTO) for ocular AEs varies by drug; enfortumab vedotin has the earliest onset at approximately 12.5 days.
How prevalent are ocular AEs in patients receiving ADC therapy?
The prevalence of ocular AEs can range from 20% to 90%, depending on the specific ADC used and patient factors.
What mechanisms are proposed for ADC-induced ocular toxicity?
Proposed mechanisms include linker instability leading to premature payload release, the bystander effect affecting nearby target antigen-negative cells, and nonspecific uptake of ADC components into normal corneal epithelial cells.
What are antibody-drug conjugates (ADCs)?
ADCs are targeted biopharmaceutical drugs that combine monoclonal antibodies with potent anti-cancer agents linked via a stable chemical linker. They aim to deliver cytotoxic drugs specifically to tumor cells, enhancing therapeutic efficacy while minimizing damage to healthy tissues. As of now, 12 ADCs have received market approval, with many more in clinical trials for various cancers.
What are the common side effects associated with ADCs?
Common side effects of ADCs include ocular adverse events such as dry eye, blurred vision, and keratopathy. Other side effects may include infusion reactions, fatigue, and gastrointestinal symptoms. The incidence and severity of these effects can vary depending on the specific ADC used.
What are the common eye problems?
Common eye problems include refractive errors such as myopia (short-sightedness), hyperopia (long-sightedness), astigmatism, and presbyopia (inability to focus on near objects). Other frequent issues are sore and tired eyes, blurred vision, dry eyes, and cataracts.
What are refractive errors?
Refractive errors occur when the eye cannot bend light at the proper angle due to an irregular eye or corneal shape1. The main types of refractive errors are astigmatism (blurry images), hyperopia (farsightedness), myopia (nearsightedness), and presbyopia (difficulty seeing up close with age)