Piotr J. Wysocki, Head of the Oncology Department at the
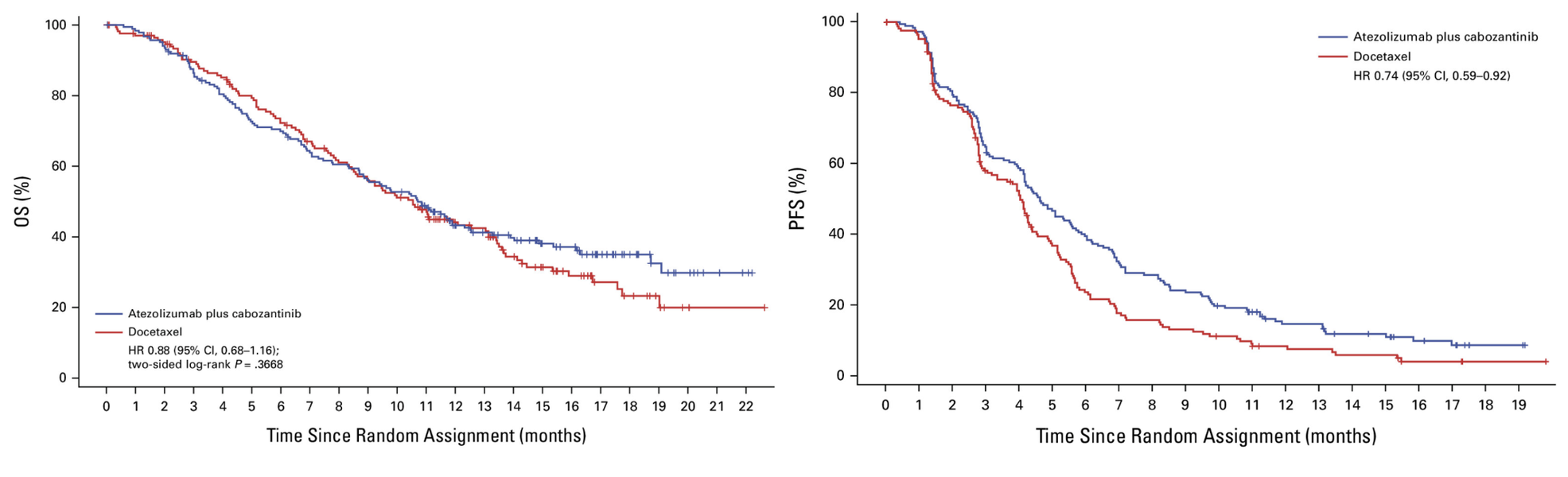
“An open-label, phase III clinical study (CONTACT-01) compared a combination of immunotherapy (anti-PD-L1) and tyrosine-kinase inhibitor (TKI) with standard chemotherapy in the second-line treatment in NSCLC patients after platinum-based chemoimmunotherapy failure. The study randomized 366 patients in a 1:1 ratio to atezolizumab+cabozantinib (investigational arm) or docetaxel (comparator)
After a median follow-up of 10.9 months, the median OS (primary endpoint) was 10.7 and 10.5 months in the investigational and docetaxel arms, respectively, which translated into an HR for death – 0.88 (95%CI 0.68-1.16)). Median PFS was 4.6 and 4.0 months, respectively (HR for PFS – 0.74; 95% CI 0.59 to 0.92). The investigational treatment demonstrated similar toxicity as docetaxel, with serious adverse events observed in 38.4% and 34.7% and with grade 3/4 AE occurring in 39.5% and 34.7% in the investigational and comparator arms, respectively.
In summary, an expensive and non-toxic innovative second-line treatment failed to improve outcomes compared to an old, well-known, single-agent docetaxel. However, there may be a population of patients (approx. 40%) who may benefit from the immunotherapy-TKI combination (a plateau of the OS curve after 16-18 months).”