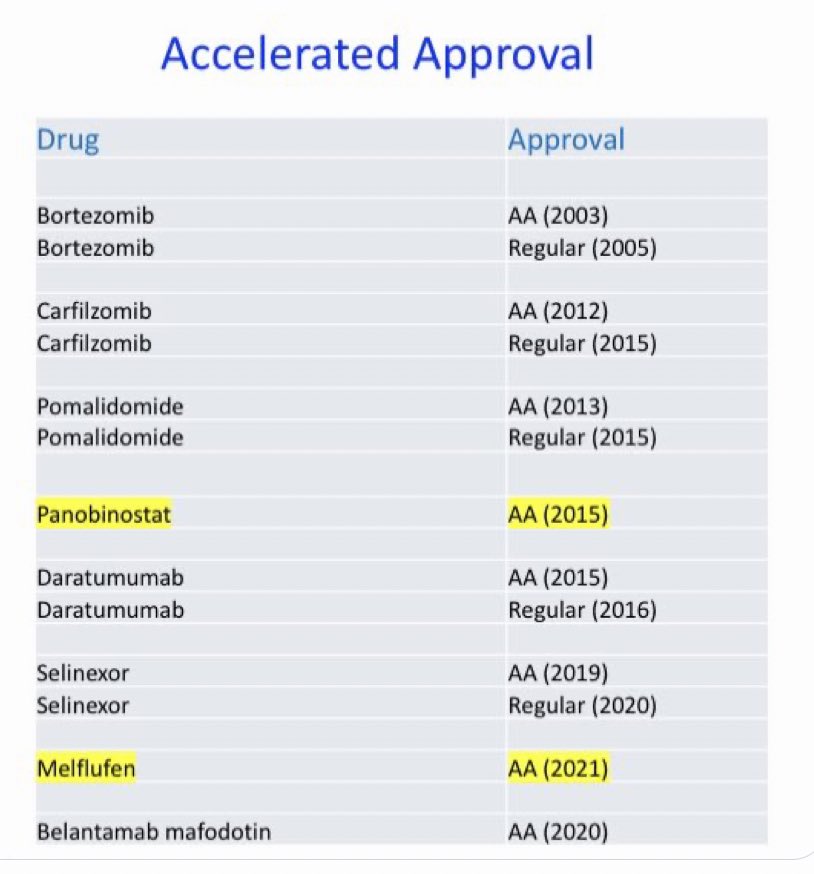
Vincent Rajkumar, Editor-in-Chief at the Blood Cancer Journal, shared a thread on X/Twitter:
“How many years of life gained because of FDA accelerated approval in myeloma? A very conservative estimate: 100,000 person years. We need this pathway:
Conservatively I assumed that bortezomib, carfilzomib, pomalidomide, and daratumumab prolonged life by median of 6 months; and only 25% of eligible patients received them. I looked at number of years we had the drug approved early compared to if we had to wait for phase III approval.
I also didn’t consider any life years gained due to selinexor, belantamab, or panobinostat. And estimated 10,000 years of life lost due to melflufen even though hardly anyone received the drug and even though the effect size is much smaller.
Estimating only a very modest benefit from all 3 bispecific approvals. ~ 6 months gained and 5-10% eligible patients having access.
We need to preserve the accelerated approval pathway for myeloma which has been of enormous benefit to patients. The years of life gained is far higher than any risk of approving an ineffective or harmful drug in myeloma. I explain more in this thread.”
Source: Vincent Rajkumar/X