
Adagrasib (Krazati) Updates 2025: Uses in Cancer, Side Effects, Dosages, Expectations, and More
Adagrasib is a novel targeted therapy designed to inhibit a specific cancer-driving mutation known as KRAS G12C—a mutation long considered difficult to treat. By selectively binding to this abnormal protein, adagrasib helps interrupt key signals that fuel tumor growth and survival. Its structure is optimized for prolonged activity in the body, offering the potential for lasting therapeutic effects.
With strong scientific rationale and encouraging data from clinical trials, adagrasib represents a major step forward in the field of precision oncology. This article will explore how adagrasib works, its potential side effects, dosage considerations, and how effective it has been in studies.
Which company produced Adagrasib?
Adagrasib, marketed as Krazati, was developed by Mirati Therapeutics, a San Diego–based biotech company specializing in precision oncology. Mirati focuses on therapies that target specific genetic mutations driving cancer, and adagrasib is one of its flagship KRAS G12C inhibitors. To expand access and accelerate development, Mirati has formed key collaborations. In 2021, it partnered with Zai Lab, granting exclusive rights to develop and commercialize adagrasib in mainland China and nearby regions. This alliance helps speed up clinical trials and regulatory approvals in Asia.
Mirati also collaborates with Sanofi, combining adagrasib with Sanofi’s SHP2 inhibitor in KRAS G12C-mutant non-small cell lung cancer. Another partnership with Kura Oncology evaluates adagrasib with Kura’s farnesyl transferase inhibitor KO-2806, aiming to enhance treatment durability. These collaborations reflect Mirati’s strategy to strengthen adagrasib’s global reach and improve outcomes through combination therapies. In January 2024, Bristol Myers Squibb completed its acquisition of Mirati, thereby bringing Mirati— and Krazati— into its oncology portfolio.
How does Adagrasib work?
Krazati is a covalent and selective inhibitor designed to target cancers harboring the KRAS G12C mutation, a genetic alteration found in several solid tumors. It works by irreversibly binding to the mutant cysteine residue at codon 12, which is unique to the KRAS G12C protein. This binding locks KRAS in its inactive GDP-bound state, thereby preventing it from switching to the active GTP-bound form that triggers cell signaling.
By keeping KRAS in its inactive form, adagrasib effectively shuts down key downstream signaling pathways, such as the MAPK/ERK and PI3K/AKT pathways, which are essential for cancer cell growth, proliferation, and survival. Its prolonged half-life and sustained target occupancy further enhance its ability to inhibit tumor growth in KRAS G12C-driven cancers.
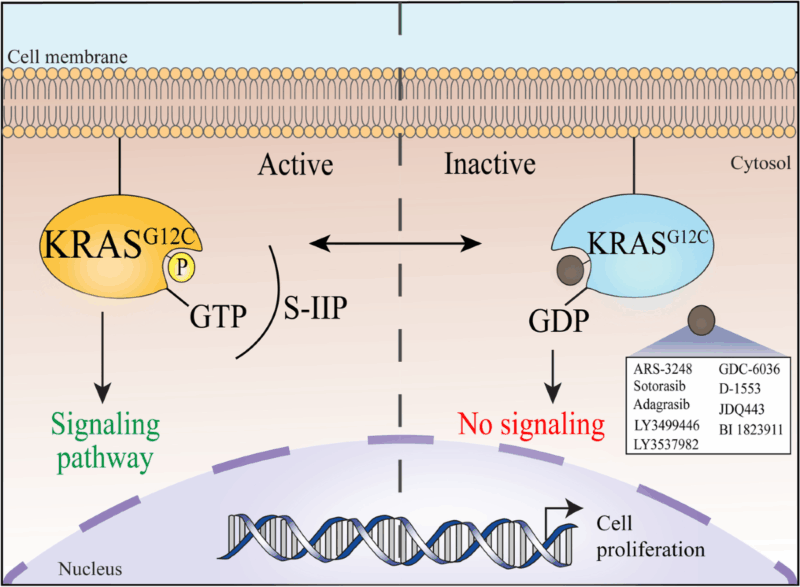
Source: Journal of Experimental & Clinical Cancer Research
What Cancers are treated with Adagrasib?
Adagrasib received U.S. FDA approval for the treatment of two cancer types. Krazati received accelerated FDA approval in December 2022 for treating adult patients with KRAS G12C-mutated advanced non-small cell lung cancer (NSCLC) who had prior systemic therapy. In June 2024, the FDA also approved adagrasib combined with cetuximab for adults with KRAS G12C-mutated locally advanced or metastatic colorectal cancer (CRC) after prior chemotherapy. Both approvals are based on response rates and duration of response, with further confirmation required.
What research is behind the approval?
Adagrasib’s approvals for non–small-cell lung cancer (NSCLC) and colorectal cancer (CRC) are based on findings from the KRYSTAL-1 clinical trial. This study evaluated adagrasib alone in patients with KRAS G12C-mutant NSCLC who had received prior treatments, and in combination with cetuximab for patients with KRAS G12C-mutated CRC after chemotherapy. The trial demonstrated adagrasib’s effectiveness and manageable safety in these specific patient groups, supporting its regulatory approvals.
Adagrasib for NSCLC
Approval for Krazati in non–small-cell lung cancer (NSCLC) is based on results from the KRYSTAL-1 phase 2 trial, which evaluated the drug in patients with previously treated KRASG12C-mutant NSCLC. In this registrational cohort, 116 patients—98.3% of whom had prior chemotherapy and immunotherapy—received adagrasib 600 mg orally twice daily. The confirmed objective response rate was 42.9%, with a median duration of response of 8.5 months and median progression-free survival of 6.5 months. Median overall survival was 12.6 months at a median follow-up of 15.6 months. Among 33 patients with stable brain metastases, the intracranial confirmed response rate was 33.3%.
Treatment-related adverse events occurred in 97.4% of patients, with 52.6% experiencing grade 1 or 2 events and 44.8% grade 3 or higher, including two treatment-related deaths (grade 5). Drug discontinuation due to adverse events was reported in 6.9% of patients. These findings, published in the New England Journal of Medicine in 2022, demonstrated adagrasib’s clinical efficacy and manageable safety profile in this biomarker-defined NSCLC population, supporting its FDA approval.
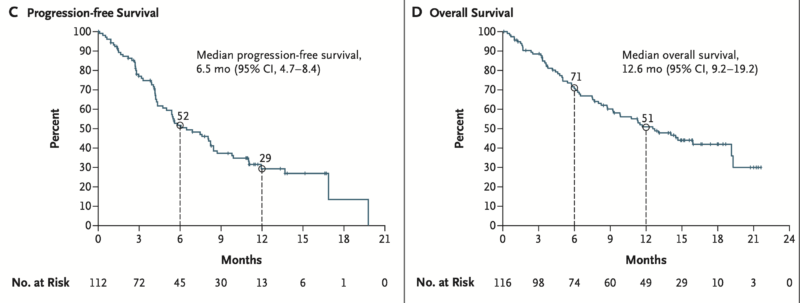
Adagrasib for CRC
The FDA approval for colorectal cancer (CRC) was based on the KRYSTAL-1 trial, which evaluated adagrasib combined with cetuximab in adults with KRAS G12C-mutated locally advanced or metastatic CRC who had previously received chemotherapy. In this study, the combination achieved a 34% overall response rate, with all responses being partial and a median duration of response of 5.8 months. Common side effects included rash, nausea, diarrhea, vomiting, fatigue, and liver-related issues. Adagrasib is administered orally at 600 mg twice daily until disease progression or unacceptable toxicity occurs. This approval was granted priority review and breakthrough therapy status, reflecting its significant clinical benefit for this patient population.
Learn more about Colorectal Cancer: Epidemiology, Pathogenesis, Diagnosis, and Therapeutic Advances on OncoDaily.
Adagrasib Side Effects and Its Management
While adagrasib has shown promising clinical benefits, it can also cause a range of side effects, some of which may require supportive care or treatment adjustments. Recognizing and managing these effects early can significantly improve patient outcomes and adherence to therapy.
Common Side Effects
The most frequently reported side effects include fatigue, nausea, vomiting, diarrhea, and decreased appetite. Many patients also experience elevated liver enzymes, such as AST and ALT, as well as anemia and dry skin or rash. Gastrointestinal side effects typically occur early during treatment and may persist throughout therapy. While most of these reactions are mild to moderate in severity, they can become more intense without proper symptom control. Monitoring lab values, especially liver function tests, is crucial during the first several weeks of treatment.
Less Common and Serious Side Effects
Although rare, Krazati has been associated with more serious toxicities. These include interstitial lung disease (ILD)/pneumonitis, hepatotoxicity, QTc interval prolongation, and gastrointestinal complications such as intestinal obstruction or colitis. Pneumonitis, if it develops, often presents with new or worsening respiratory symptoms and requires immediate evaluation. Prolongation of the QT interval can predispose patients to cardiac arrhythmias and must be monitored with regular electrocardiograms, particularly in those with baseline cardiac risk factors or those taking other QT-prolonging drugs. Hepatic injury, while often asymptomatic at first, can lead to treatment interruptions if enzyme elevations become significant.
Management Strategies
Effective management begins with early recognition. Nausea and vomiting can usually be controlled with antiemetics. Diarrhea is often manageable with loperamide and fluid support. Fatigue should prompt evaluation for anemia or nutritional deficiencies, and appropriate interventions such as iron supplementation or energy-conserving strategies may be considered. For rash and dry skin, topical corticosteroids and moisturizers are often helpful.
In patients with significant elevations in liver enzymes, dose interruption or reduction may be required, followed by careful rechallenge once levels normalize. QTc prolongation should prompt electrolyte correction, medication review, and cardiology consultation if needed. If interstitial lung disease or pneumonitis is suspected, adagrasib should be withheld immediately and corticosteroids initiated if confirmed. In such cases, permanent discontinuation may be necessary. Regular monitoring and open communication between healthcare providers and patients are key to managing adagrasib-related side effects and maintaining therapy over the long term.

What is the Recommended Dosage of Adagrasib?
Krazati is available as 200 mg tablets and is approved for KRAS G12C–mutated locally advanced or metastatic NSCLC in adults who have received at least one prior systemic therapy. The recommended dose is 600 mg taken orally twice daily until disease progression or unacceptable toxicity. For KRAS G12C–mutated metastatic colorectal cancer (CRC), adagrasib is used in combination with cetuximab after prior fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy. Cetuximab may be given at 400 mg/m² IV once, followed by 250 mg/m² weekly, or 500 mg/m² every two weeks.
In case of side effects, dose reductions may be needed—first to 400 mg twice daily, and then to 600 mg once daily. If the lowest dose isn’t tolerated, treatment should be discontinued. Adagrasib may require interruption or dose adjustment for severe nausea, diarrhea, elevated liver enzymes, QT prolongation, or interstitial lung disease. Liver function and heart rhythm (ECG) should be monitored regularly, especially in patients with risk factors. No dose adjustment is needed for patients with kidney or liver impairment. Testing for KRAS G12C mutations is required before starting treatment.
How is Adagrasib administered?
Krazati should be taken by mouth at the same time each day, with or without food. Tablets must be swallowed whole—do not chew, crush, or split them. If a dose is missed and more than four hours have passed, skip it and take the next dose as scheduled. If vomiting occurs after taking Krazati, do not retake the dose. Store the medication at room temperature (20–25°C), with short-term excursions between 15–30°C allowed.
Learn more about Non-Small Cell Lung Cancer: Causes, Symptoms, Diagnosis, Treatment Options, and Latest 2025 Advances in Targeted and Immunotherapy on Oncodaily.
How Is Adagrasib Metabolized and Eliminated?
Adagrasib is highly protein-bound and widely distributed in the body. It’s mainly broken down by the liver enzyme CYP3A4, though with repeated dosing, other enzymes also contribute. The drug has a half-life of about 23 hours and is cleared from the body at a rate of 37 L/hour. Most of it is excreted in the feces, with a small amount eliminated through urine.
What to Avoid During Adagrasib Treatment?
While taking adagrasib, patients should avoid certain substances and actions that may interfere with the drug’s effectiveness or increase side effects. Strong CYP3A4 inducers (such as rifampin or St. John’s wort) should be avoided, as they can reduce adagrasib levels in the body. Similarly, grapefruit or grapefruit juice may affect how the drug is metabolized and should not be consumed.
Patients should also avoid smoking, as it can alter liver enzyme activity and potentially affect drug metabolism. It’s important not to take other medications or supplements without discussing them with your doctor, as interactions are possible. Additionally, because adagrasib may cause fatigue or dizziness, caution is advised when driving or operating heavy machinery until you know how the drug affects you.
Adagrasib effectiveness over time
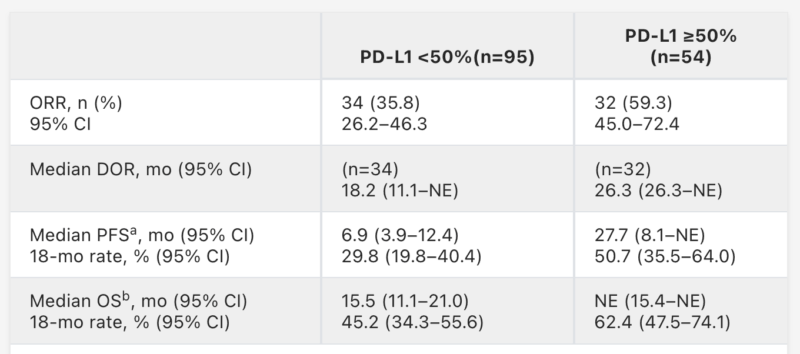
An updated analysis from the Phase 2 KRYSTAL-7 trial, published in the Journal of Clinical Oncology (May 2025), highlighted the long-term effectiveness of adagrasib combined with pembrolizumab as a first-line treatment for advanced or metastatic KRAS G12C–mutated non-small cell lung cancer (NSCLC). This was the first study to evaluate this combination across all PD-L1 expression subgroups.
Patients received Krazati 400 mg twice daily plus pembrolizumab every 3 weeks. Among 150 response-evaluable patients, the confirmed objective response rate (ORR) was 49%, with 3 complete responses. Responses were observed across all PD-L1 expression levels (≥50%, 1–49%, and <1%). At a median follow-up of 15.3 months, the median duration of response (DoR) was 12.6 months, and median progression-free survival (PFS) was 8.3 months. The median overall survival (OS) reached 18.1 months.
These results confirm durable antitumor activity and a manageable safety profile, supporting adagrasib plus pembrolizumab as a promising frontline option. A randomized phase 3 trial comparing this combination to pembrolizumab alone is underway to further validate these findings.
Ongoing trials with Adagrasib
The KRYSTAL-21 is a Phase 2 trial sponsored by Mirati Therapeutics that compares two dosing schedules of adagrasib—600 mg twice daily without regard to food versus 400 mg twice daily with food—in patients with KRAS G12C-mutated non-small cell lung cancer (NSCLC). Eligible patients have previously been treated with platinum-based chemotherapy and immune checkpoint inhibitors. The study aims to assess which dosing regimen offers better efficacy and tolerability in this population.
Written by Mariam Khachatryan, MD
FAQ
What is a KRAS G12C mutation, and why is it important in cancer?
KRAS G12C is a genetic mutation that drives cancer growth, seen in lung and colorectal cancers. It was hard to treat until KRAS inhibitors like adagrasib were developed.
How does adagrasib differ from sotorasib?
Both target KRAS G12C, but adagrasib has a longer half-life and different dosing. Studies are ongoing to compare long-term outcomes.
What should patients avoid while taking Krazati?
Avoid CYP3A4 inducers, grapefruit, smoking, and unapproved medications or supplements due to interaction risks.
What is the approved dosage of adagrasib?
The recommended dose is 600 mg orally twice daily for both NSCLC and colorectal cancer. Dose adjustments may be needed for side effects.
What is the half-life of adagrasib?
Adagrasib has a half-life of approximately 23 hours.
What are common side effects of Krazati?
Common side effects include nausea, diarrhea, vomiting, fatigue, and liver enzyme elevations. Serious risks include pneumonitis, QT prolongation, and hepatotoxicity.
How is adagrasib administered?
Adagrasib is taken by mouth, twice daily, with or without food. Tablets must be swallowed whole—do not chew, split, or crush them.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
