Jean-Charles Soria, Head of Oncology Therapeutic Area at Amgen, shared a recent article by Mathias Baedeker et al. on X:
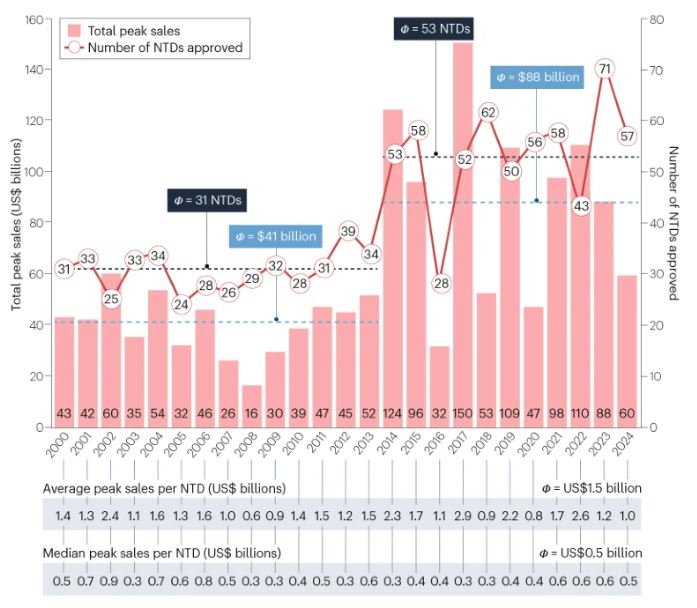
“FDA approved 57 new therapeutic drugs (NTDs) in 2024. This is above the average of 53 NTDs per year for the last decade and below the 71 approvals in 2023.
The total projected annual peak sales for these NTDs is US$60 billion, which is considerably below the average since 2014 of $88 billion.”
“2024 FDA approvals exceed average number but have lower sales projections“
Authors: Mathias Baedeker, Michael S. Ringel and Clemens C. Möller
Jean-Charles Soria serves as Amgen’s senior vice president of Oncology within Global Development. From 2017 to 2019, he was the senior vice president of Research and Development in Oncology at AstraZeneca. Additionally, Soria has authored or co-authored over 670 articles in leading international journals and has been recognized as one of the world’s most influential research scientists.
More posts featuring Jean-Charles Soria.


