Sumanta K. Pal, Co-Director of the City of Hope Kidney Cancer Program, shared on X/Twitter:
“Very excited to share with you incredibly promising data for Zanzalintinib (XL092) in patients with kidney cancer. A million thanks to the Kidney Cancer Association for allowing us to present this data in a highlighted oral session at IKCSNA23 (International Kidney Cancer Symposium: North America)!
As background, Zanzalintinib is a multikinase inhibitor with affinity for VEGR, MET and TAM kinases. Short t(1/2) may improve tolerability. We have previously reported data from a dose escalation study (ESMO22). We report at IKCSNA23 a dedicated ccRCC expansion cohort.

A total of 32 patients with ccRCC were enrolled. Pts had 1-3 prior systemic anticancer therapies; sarcomatoid features were permitted. We report safety in a cohort of 81 pts, inclusive of those enrolled in dose escalation.
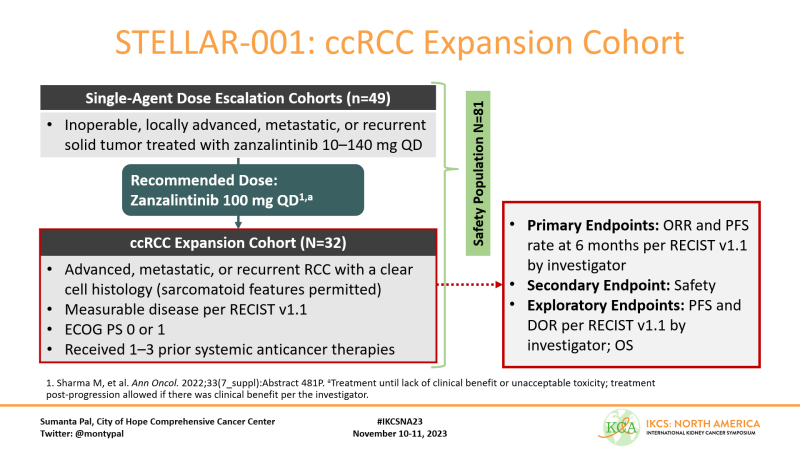
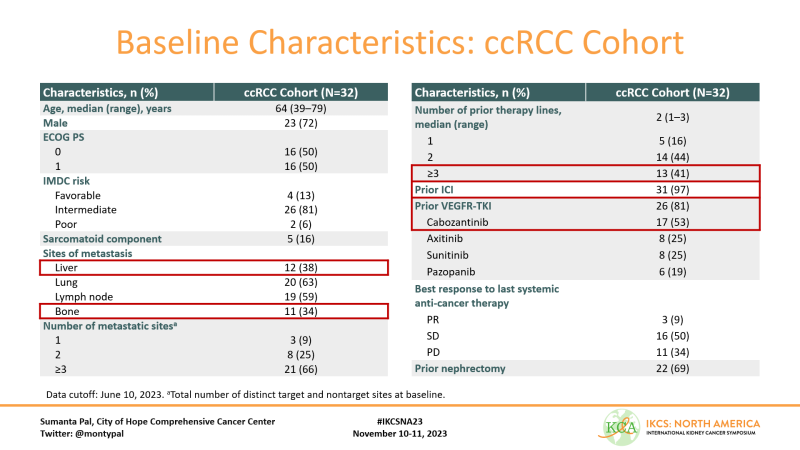
Important to keep in mind pts in this cohort had an aggressive phenotype (38% & 34% w liver and bone mets, respectively) and 41% had 3 prior lines of treatment. Almost all had prior ICI and 81% had prior TKI, incl 53% with prior Cabozantinib.
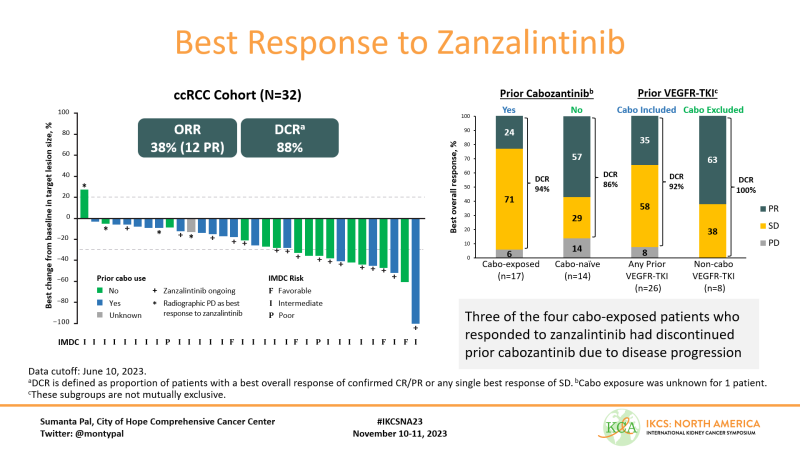
I was impressed with the ORR of 38% & DCR of 88%. Moreover, see the panel on the right – RR of 63% in patients who were cabozantinib naive and prior TKI!!! Responses also observed in pts with prior cabo – 3 of 4 responses were in patients with disease prog on cabo.
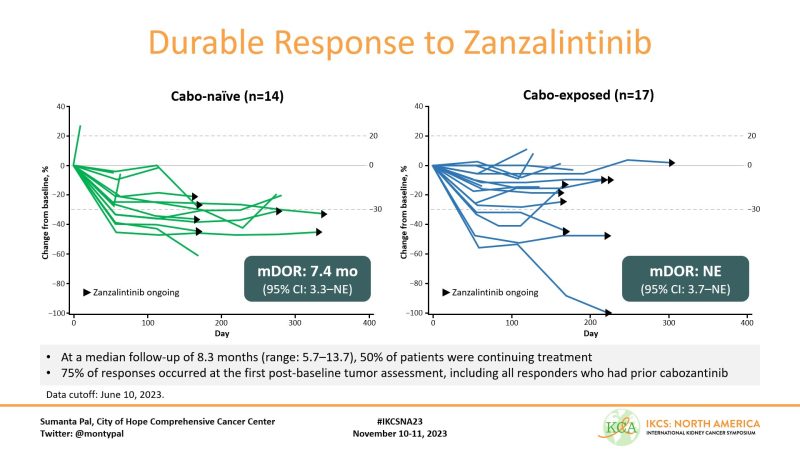
Many responses ongoing – will be of interest to see how this data matures. mDOR was 7.4 mos in cabo-naive patients and not reached in cabo-exposed. Importantly, responses came early – typically at time of first post-baseline assessment.
Safety is an area where this agent stands out IMHO. Rates of G3 diarrhea, asthenia, stomatitis and PPE far lower than what I’m used to in this setting. I’ve had patients on Zanzalintinib who did not tolerate other TKIs (including Cabozantinib) who did well on this agent.
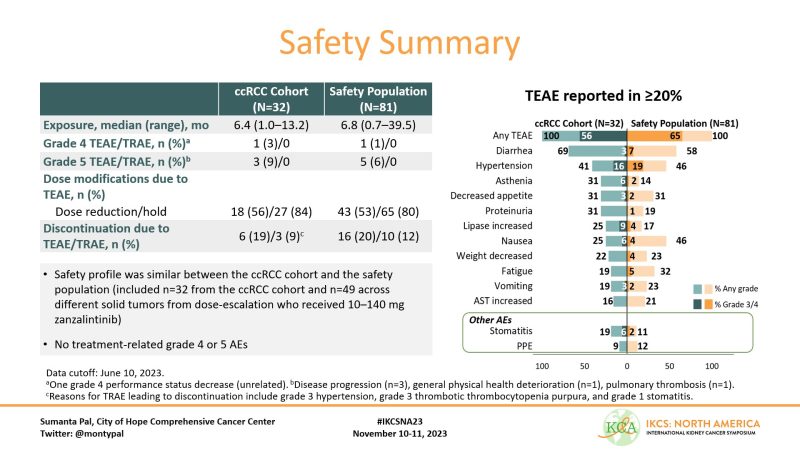
In summary, Zanzalintinib is different – potent activity after multiple prior TKIs, including cabo. Tox profile also distinguishes it from other agents. So where do we go from here?
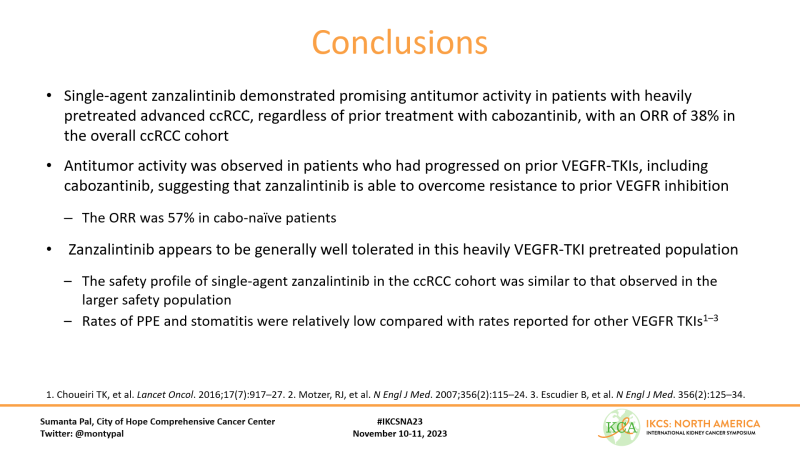
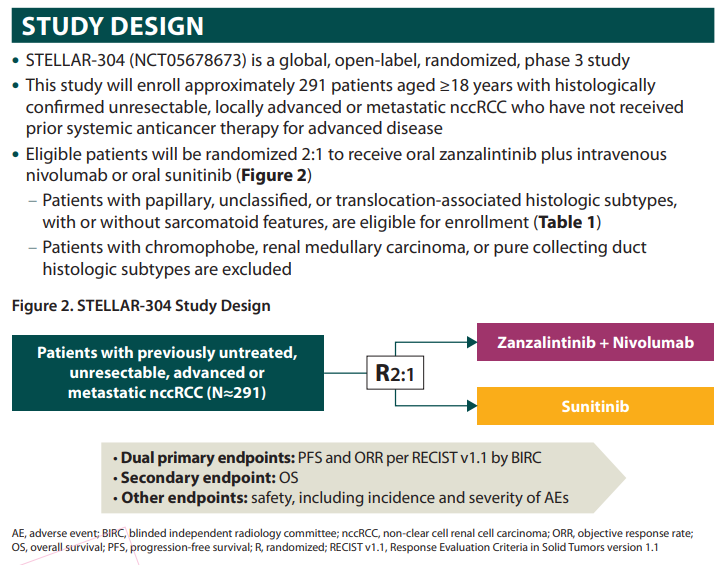
LOTS more to come with Zanzalintinib across tumor types. My personal plea is that you consider STELLAR304, a study that will hopefully introduce a new standard for non-clear cell kidney cancer. Come by our poster here at IKCSNA23!
Source: Sumanta K. Pal/Twitter
