Ziihera is a targeted treatment used to treat HER2-positive biliary tract cancer (BTC) that has already been treated but has spread to other parts of the body (metastasized) or cannot be removed by surgery. Ziihera is a bispecific HER2-directed antibody, which means it targets two cancer-causing proteins (HER2) on the tumor cells. Ziihera is not a chemotherapy. Ziihera (zanidatamab-hrii) is given as an intravenous infusion once every 2 weeks.
Ziihera FDA approval was granted for the treatment of adults who have a type of bile duct (cholangiocarcinoma) or gallbladder cancer called biliary tract cancer (BTC), which has been confirmed to be human epidermal growth factor receptor 2 (HER2)-positive (IHC 3+). The biliary tract cancer must have been previously treated and is not able to be removed by surgery or has spread to other parts of your body (metastatic).
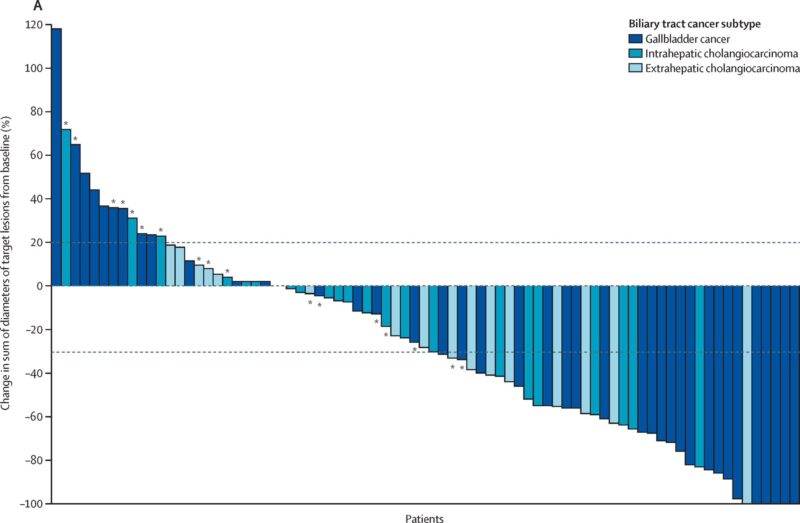
Ziihera FDA approval was based on positive results from the HERIZON-BTC-01, which showed an objective response rate (ORR) of 52% with a median duration of response (DOR) of 14.9 months. Ziihera was approved under accelerated approval, and continued approval for this indication is dependent on clinical benefit in further trial results.
Mechanism of Action
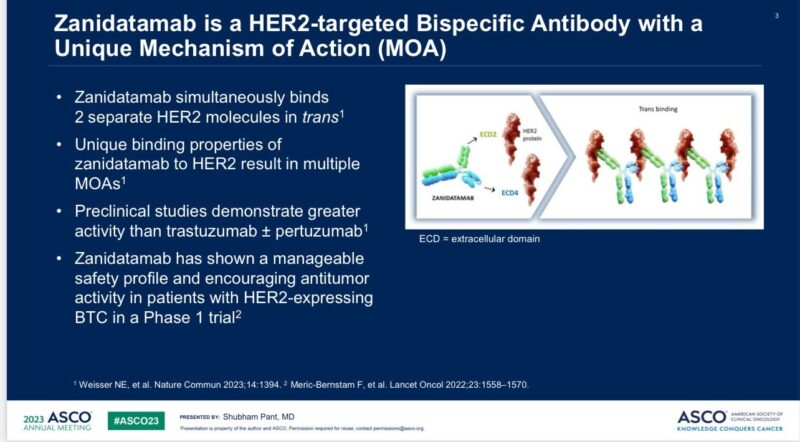
Bispecific human epidermal growth factor receptor-2 (HER2)-directed monoclonal antibody that targets 2 non-overlapping epitopes on same target, extracellular domain 4 (ECD4) and ECD2
HER2 overexpression may occur in some biliary tract cancers
Binding to HER2 expressed on surface of cancer cells results in cell internalization leading to reduction of tumor cell surface receptors
Tumor growth inhibition and cell death occur due to complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP), as demonstrated in vitro and in vivo studies
Ziihera side effects
Common Ziihera side effects
Common Ziihera side effects are
- diarrhea (50%)
- infusion-related reactions (35%)
- stomach pain (29%)
- nausea (18%)
- vomiting (15%)
- tiredness (24%)
- rash (19%)
- reduced appetite (16%).
These side effects occurred in 15% or more patients in a clinical trial. Ziihera can also affect laboratory results, causing reduced hemoglobin and lymphocytes and other laboratory abnormalities.
Serious side effects and warnings
Ziihera may cause serious side effects, including:
Left Ventricular Dysfunction: a decrease in left ventricular ejection fraction (LVEF) may occur in some patients, assess left ventricular ejection fraction (LVEF) before starting treatment and at regular intervals during treatment.
Infusion-Related Reactions (IRRs): IRR were reported in 31% of 233 patients treated with Ziihera as a single agent in clinical studies. Premedicate before each infusion. Stop the infusion, decrease the infusion rate, and/or permanently discontinue based on infusion-related reactions severity.
Toxic to an unborn baby: Based on the way Ziihera works it can cause fetal harm when administered to a pregnant woman. Females who are able to become pregnant should use birth control (contraception) during treatment with Ziihera and for 4 months after the last dose.
Dosage and Administration: The recommended dose is Ziihera 50 mg/mL, administered intravenously. Its continued approval is subject to further verification of clinical benefits in ongoing trials.
Authors: Shubham Pant, Jia Fan, Do-Youn Oh, Hye Jin Choi, Jin Won Kim, Heung-Moon Chang, Lequn Bao, Hui-Chuan Sun, Teresa Macarulla Mercade, Feng Xie, Jean Philippe Metges, Ying Jieer, John A Bridgewater, Mohamedtaki Abdulaziz Tejani, Emerson Yu-sheng Chen, Harpreet Singh Wasan, Michel Pierre Ducreux, Jia-Fang Ma, Phillip M. Garfin, and James J. Harding

The Principal Investigator (PI) for this trial is Dr. Shubham Pant, MD.
Shubham Pant is a Professor in the Department of Gastrointestinal (GI) Medical Oncology with a joint appointment in the Department of Investigational Cancer Therapeutics (Phase I Center) at The University of Texas MD Anderson Cancer Center.
Shubham Pant specialises in the treatment of Gastrointestinal Cancers with an emphasis on Pancreatic and Biliary cancers and Phase 1 trials. His research focuses on novel immunotherapeutic approaches and targeted therapies in GI cancers, including devising novel ways to target the KRAS mutation.
He has helped draft the American Society of Clinical Oncology (ASCO) Metastatic Pancreatic Cancer Guidelines. Dr. Pant completed his fellowship from the James Cancer Hospital/Solove Research Institute at the Ohio State University. Dr. Pant has received numerous awards and honors including the Golden Pillar Award for Outstanding Patient Service and the Mai Eager Anderson Endowed Chair in Cancer Clinical Trials.

Oncologists and Organizations shared their opinion on it.
Amol Akhade, Senior Consultant Medical Oncologist and Hemato-oncologist at Suyog Cancer Clinics and Reliance Hospitals, shared a post on LinkedIn:
“Zanidatamab, Her2 bispecific, got accelerated approval by US FDA for advanced BTC with IHC 3 plus for second line.
Based upon 52 % of ORR and DOR of 14.9 months in phase 2 trial.
Will it stand in phase 3 trial? Let’s hope so.”
Tanios Bekaii-Saab, Medical Onclogist at Mayo Clinic, shared on X:
“Another win for cholangiocarcinoma patients! Transforming the treatment landscape one target at a time over just one decade! ”
Pashtoon Kasi, Medical Director of GI Medical Oncology at City of Hope, shared a post on X:
“New Drug approval!
Precision Medicine.
HER2-targeted bispecific antibody.
Zanidatamab-hrii for HER2(IHC 3+) Biliary Tract Cancer (BTC).
Trials possible for ‘rare’ cancers like cholangiocarcinoma. More options for our patients.“
Rohit Gosain, Co-Host of Podcast Oncology Brothers, shared on LinkedIn:
“Zanidatamab (bispecific antibody against Her2) now FDA approved for unresectable/metastatic HER2+ biliary tract cancer based off HerizonBTC01:- Single arm Ph2B study, 20 mg/kg IV Q2W- mDoR 14.9mos- mOS 15.5mos- AEs: Diarrhea, decreased EF. Shubham Pant et al. congratulations!!”
For more information visit oncodaily.com


