On October 18, 2024, the Food and Drug Administration approved zolbetuximab-clzb (Vyloy, Astellas Pharma US, Inc.), a claudin 18.2 (CLDN18.2)-directed cytolytic antibody, with fluoropyrimidine- and platinum-containing chemotherapy, for the first-line treatment of adults with locally advanced unresectable or metastatic human epidermal growth factor receptor 2 (HER2)-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumors are CLDN18.2 positive, as determined by an FDA-approved test.
Zolbetuximab is a monoclonal antibody specifically designed to target CLDN18.2, a tight junction protein that is overexpressed in certain types of gastric and gastroesophageal junction (GEJ) cancers.
Mechanism of Action: By binding to CLDN18.2, Zolbetuximab triggers immune responses that can lead to cancer cell death and enhance the effectiveness of chemotherapy.
It is primarily used for patients with advanced or metastatic gastric adenocarcinoma that is CLDN18.2-positive and HER2-negative.
Efficacy was evaluated in trials SPOTLIGHT (NCT03504397) and GLOW (NCT03653507). Both were randomized (1:1), double-blind, multicenter trials that enrolled patients with CLDN18.2 positive advanced unresectable or metastatic HER2-negative gastric or GEJ adenocarcinoma. The major efficacy outcome measure in both trials was progression-free survival (PFS), as assessed per RECIST v1.1 by an independent review committee. Overall survival (OS) was an additional efficacy outcome measure.
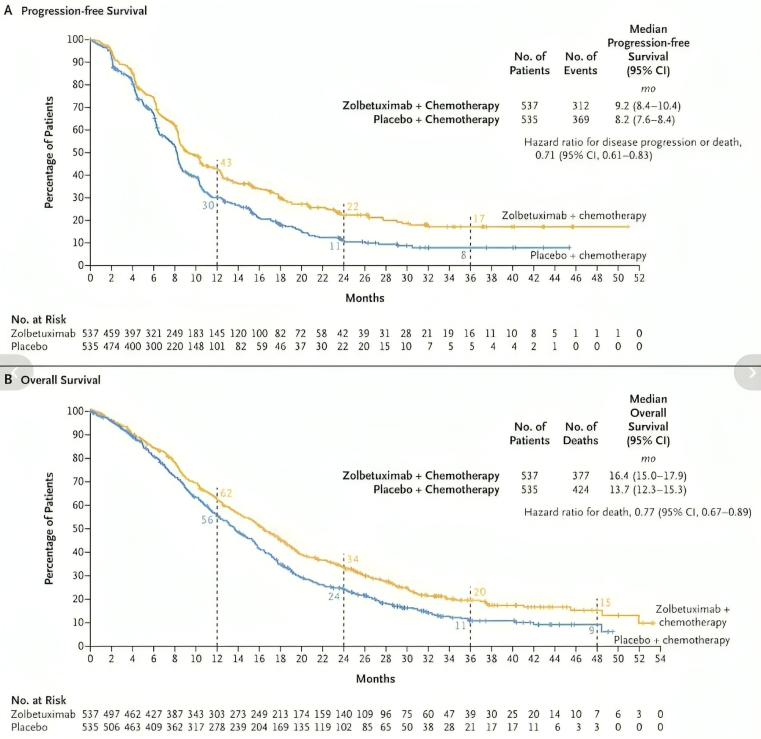
In SPOTLIGHT, 565 patients were randomized to receive zolbetuximab-clzb with mFOLFOX6 chemotherapy or placebo with mFOLFOX6 chemotherapy. Median PFS was 10.6 months (95% CI: 8.9, 12.5) in the zolbetuximab-clzb/chemotherapy arm and 8.7 months (95% CI: 8.2, 10.3) in the placebo/chemotherapy arm (hazard ratio [HR] 0.751 [95% CI: 0.598, 0.942]; 1-sided p-value=0.0066). Median OS was 18.2 months (95% CI: 16.4, 22.9) and 15.5 months (95% CI: 13.5, 16.5), respectively, (HR 0.750 [95% CI: 0.601, 0.936]; 1-sided p-value=0.0053).
The most common serious adverse reactions in SPOTLIGHT (≥2%) were vomiting, nausea, neutropenia, febrile neutropenia, diarrhea, intestinal obstruction, pyrexia, pneumonia, respiratory failure, pulmonary embolism, decreased appetite, and sepsis. The most common serious adverse reactions in GLOW (≥2%) were vomiting, nausea, decreased appetite, decreased platelet count, upper gastrointestinal hemorrhage, diarrhea, pneumonia, pulmonary embolism, and pyrexia.
The recommended zolbetuximab-clzb dosage with fluoropyrimidine- and platinum-containing chemotherapy is:
- First dose: 800 mg/m2 intravenously,
- Subsequent dosages:
- 600 mg/m2 intravenously every 3 weeks, or
- 400 mg/m2 intravenously every 2 weeks.
Authors: Kohei Shitara, Florian Lordick, Yung-Jue Bang, Peter Enzinger, David Ilson, Manish A Shah, Eric Van Cutsem, Rui-Hua Xu, Giuseppe Aprile, Jianming Xu, Joseph Chao, Roberto Pazo-Cid, Yoon-Koo Kang, Jianning Yang, Diarmuid Moran, Pranob Bhattacharya, Ahsan Arozullah, Jung Wook Park, Mok Oh, Jaffer A Ajani
The principal investigator for the trial is Kohei Shitara.

Dr Kohei Shitara is the Director of the Department of Gastrointestinal Oncology, National Cancer Center Hospital East, in Japan. Main research interests include development of new anti-cancer agents, optimal chemotherapy regimen for gastrointestinal cancer, and translational research.
He is the primary investigator or study coordinator of several sponsor-initiated or investigator-initiated trials for gastric cancer. He was recognised as one of Highly Cited Researchers by Clarivate in 2022 and 2023.
In GLOW, 507 patients were randomized to receive either zolbetuximab-clzb with CAPOX chemotherapy or placebo with CAPOX chemotherapy. Median PFS was 8.2 months (95% CI: 7.5, 8.8) in the zolbetuximab-clzb/chemotherapy arm and 6.8 months (95% CI: 6.1, 8.1) in the placebo/chemotherapy arm (hazard ratio [HR] 0.687 [95% CI: 0.544, 0.866]; 1-sided p-value=0.0007). Median OS was 14.4 months (95% CI: 12.3, 16.5) and 12.2 months (95% CI: 10.3, 13.7), respectively (HR 0.771 [95% CI: 0.615, 0.965]; 1-sided p-value=0.0118).
Authors: Carsten U Niemann,Talha Munir, Carol Moreno, Carolyn Owen, George A Follows, Ohad Benjamini, Ann Janssens, Mark-David Levin, Tadeusz Robak, Martin Simkovic, Sergey Voloshin, Vladimir Vorobyev, Munci Yagci, Loic Ysebaert, Keqin Qi, Qianya Qi, Pierre Sinet, Lori Parisi, Srimathi Srinivasan, Natasha Schuier, Kurt Baeten, Angela Howes, Donne Bennett Caces, Arnon P Kater
The principal investigator for the trial is Carsten Niemann.

Carsten Niemann, MD, PhD, is a Clinical Associate Professor and Principal Investigator at Rigshospitalet, Copenhagen, Denmark.
Dr Niemann received his medical degree in 2000 from Kobenhavns University, before completing a PhD in 2006. He then worked at Rigshospitalet, Copenhagen, Denmark, and Roskilde Sygehus, Roskilde, Denmark, until 2010. He then became a fellow in Hematology at Rigshospitalet.
Meanwhile, he also worked as the medical secretary for the Danish Medicines Agency, Copenhagen, Denmark. Then Dr Niemann worked for the National Institutes of Health and the Danish Cancer Society, Copenhagen, Denmark, until 2015 where he resides today as a consultant at Rigshospitalet.
Doctors and healthcare organisations shared their insights on social media.
“Zolbetuximab now US FDA approved — SPOTLIGHT/GLOW: ESMO24 Update, Ph III, Advanced/metastatic Gastric/GEJ w/CLDN18.2, Her2 -ve, 1L + Chemo vs chemo:
– mPFS: 9.2mos vs 8.2mos (HR: 0.71)
– mOS: 16.4mos vs 13.7mos (HR: 0.77)
– Nausea/Vomiting common SE.”
“The US FDA approved the targeted therapy zolbetuximab-clzb with chemotherapy as the first-line treatment for certain adults with HER2-negative gastric or gastroesophageal junction adenocarcinoma whose tumors are CLDN18.2 positive:”

“Exciting news! FDA approves Zolbetuximab for advanced gastric/GEJ cancer. SPOTLIGHT and GLOW trials show median OS: 16.4 months median PFS: 9.2 months. A game-changer for patients!”
“Zolbetuximab plus chemotherapy gets approval for first line Gastric or GEJ cancer with her2 negative and Claudin 18.2 positive tumors. This is based upon spotlight trial. It was approved in Japan earlier this year and approval in USA was delayed due to quality control issues.

“Zolbetuximab + chemotherapy gets FDA approval for treatment for HER2 negative gastric and GEJn adenocarcinoma in the first line setting with benefit in both PFS and OS.
Click here.”



