Steve Harvey, CEO at Camena Bioscience, shared on LinkedIn:
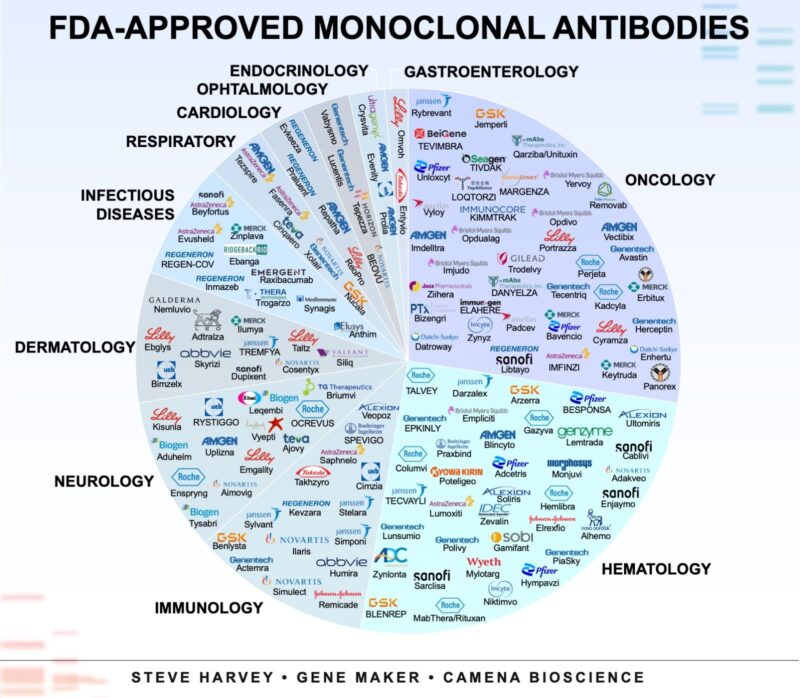
“Ever wondered about monoclonal antibody applications? The image contains every FDA-approved monoclonal antibody arranged by therapeutic areas.
Bi- and tri-specific antibodies are hot topics, but classical monoclonals (mAbs) are the workhorses of modern biotherapeutics.
In 1975, Georges Köhler and César Milstein, working in Cambridge, unlocked a new era of medicine with the development of the hybridoma technique.
That was the first method for mass-producing mAbs.
The first therapeutic mAb (Orthoclone OKT3) was approved in 1986, but there are now >100 FDA-approved mAbs.
- Oncology and haematology remain dominant applications of mAbs.
- Neurology and dermatology are growing fast with CGRP blockers and IL-23/IL-17 inhibitors.
- Interestingly, nearly 40 % of approvals are outside of cancer. I thought it would have been less.
Do you think bi-specifics will catch up?
Data source: The Antibody Society.
Some antibodies are approved for multiple therapeutic areas, and commercial rights may vary across jurisdictions or under specific licensing agreements.”
Jean-Pierre Armand, Chairman of the Board at PEGASCY, shared Steve Harvey’s post:
“MOAB
Oncology a good start for new drugs in general pathology :
nearly 40 % of approvals are outside of cancer. It will be true for discarded small molecules to morrow.”
More posts featuring Steve Harvey.


