Sergio Cifuentes Canaval, ASCO International Development and Education Award (IDEA) Recipient – 2025 at Conquer Cancer, the ASCO Foundation, shared on LinkedIn:
“SERENA-6: Unpacking the Economic Reality of ESR1m Surveillance in Breast Cancer

As an oncologist interested in breast cancer, I’m analyzing the nuances of new research, especially through the lens of global healthcare disparities. SERENA-6 study (the context of ESR1m surveillance in first-line AI+CDK4/6i), highlighted some intriguing statistics on emerging ESR1 mutations (ESR1m), 42% of patients developing emerging ESR1m during first-line AI+CDK4/6i treatment – might seem striking, a deeper dive reveals significant economic implications, particularly for healthcare systems in Latin America and other resource-constrained settings.
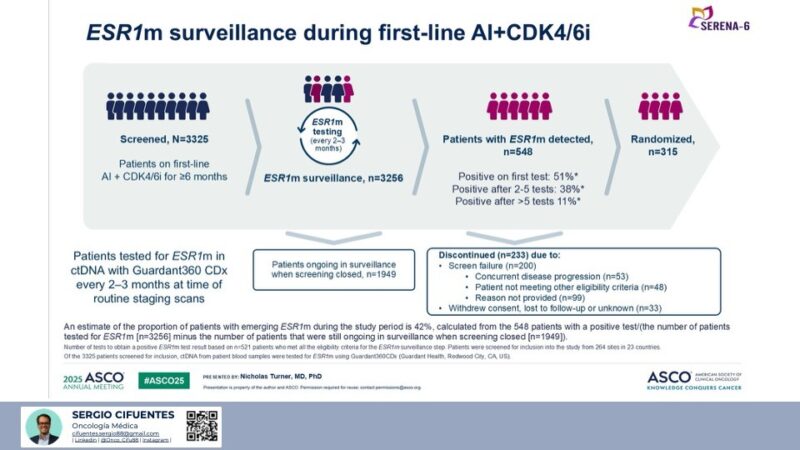
The data suggests that out of a specific cohort of 1307 patients who completed surveillance or discontinued for reasons other than being ‘ongoing’ when screening closed, 548 developed an ESR1m, leading to the 42% emergence rate. This high prevalence underscores the biological reality of adaptive resistance.
However, the devil is in the details of detection and cost. The slide indicates that among the 548 patients who eventually developed an ESR1m:
- Only 51% were positive on their first test. This translates to approximately 279 patients whose mutation was found on the initial test.
- A substantial 49% (38% after 2-5 tests, 11% after >5 tests) required multiple serial tests to detect the mutation. This means nearly half of all detected mutations were not apparent on the first screening.
When we consider the broader cohort, approximately 3256 patients were tested for ESR1m. Based on our calculations, only about 8.58% of these patients had a positive result on their first test. This implies that for a significant majority, the first test was negative, necessitating repeated, costly surveillance.
Analysis of ESR1m detection within the ‘Patients with ESR1m detected’ group (n=548):
- Positive on first test: 51%
- Positive after 2-5 tests: 38%
- Positive after >5 tests: 11%
Proportion of patients who were positive on their first test out of all patients tested for ESR1m: If we consider the 3256 patients who were ‘tested for ESR1m” as the relevant ‘at risk’ or ‘intention-to-treat (ITT)’ population for receiving at least one test, then:
279.48 test patients / 3256 test patients = 0.0858 = 8.58
Therefore, approximately 8.58% of all patients tested for ESR1m had their mutation detected on their first test.
The Economic Toxicity of Frequent Surveillance:
This is where the “fine print” becomes a major concern. The proposed surveillance frequency is “every 2-3 months” using a liquid biopsy platform like Guardant360 CDx. Let’s consider the economic burden:
- High Cost Per Test.
- Cumulative Costs: Administering these tests every 2-3 months over an extended period (patients often stay on first-line AI+CDK4/6i for 2 to 5 years) results in astronomical cumulative costs per patient. If nearly half of detected mutations require multiple tests, this further drives up the expense without immediate actionable insights.
- Limited Immediate Actionability in Many Settings: In many Latin American countries, the identification of an ESR1m might not immediately translate into a change in treatment, primarily due to: Availability of targeted therapies: Access to novel drugs for ESR1m-positive disease (like oral SERDs) is often limited or requires patient participation in clinical trials, which are not universally accessible. Resource constraints: Budgetary limitations in public health systems mean that funds for such expensive surveillance might divert resources from more fundamental cancer care, like improving access to basic diagnostics, chemotherapy, or supportive care.
- Logistical Burdens: Beyond the test itself, there are costs associated with sample collection, transportation (especially across multiple sites and countries), laboratory processing, and expert interpretation – all adding layers of expense and complexity.
A Call for Pragmatism and Equitable Care:
While the scientific advancement of detecting emerging resistance mutations is exciting, we must critically evaluate its real-world applicability and economic feasibility, particularly outside of well-resourced environments. For countries in LATAM, advocating for widespread, frequent, and expensive ESR1m surveillance without clear, immediately actionable, and accessible follow-up therapies could lead to significant financial toxicity for both patients and strained healthcare systems.
Instead, our focus in regions with limited resources should prioritize:
- Ensuring access to standard-of-care treatments.
- Investing in foundational diagnostics.
- Developing region-specific, cost-effective research strategies.
- Participating in clinical trials that offer access to innovative therapies and contribute to local data.
The 42% emergence rate of ESR1m is a vital piece of information for future drug development and understanding resistance. However, before routine clinical implementation of such surveillance, we need robust evidence demonstrating not only clinical benefit but also its cost-effectiveness and true impact on patient outcomes in diverse global health contexts. Otherwise, we risk perpetuating health disparities under the guise of advanced care.”


