Akshaya Keerti shared on LinkedIn:
“New advances in cancer cachexia management!
Ponsegromab, a GDF-15 inhibitor, shows promising results in reversing weight loss and improving physical activity in patients with advanced cancer. Check out my latest blog post on how this breakthrough could change the way we manage cancer-related muscle wasting.
Ponsegromab: A New Hope for Managing Cancer Cachexia
Cachexia, a debilitating syndrome associated with advanced malignancies, affects a significant percentage of cancer patients, leading to profound weight loss, muscle wasting, and reduced physical function. Despite nutritional interventions, this condition often worsens quality of life, reduces treatment tolerance, and increases mortality. Recent advances have focused on targeting the molecular drivers of cachexia, especially the role of Growth Differentiation Factor-15 (GDF-15), making this cytokine a key target for new therapies like Ponsegromab.
GDF-15 and Cancer Cachexia
GDF-15 is a stress-induced cytokine involved in anorexia and muscle wasting through its interaction with the Glial cell-derived neurotrophic factor receptor alpha-like (GFRAL), located in the hindbrain. Elevated GDF-15 levels in patients with cancer cachexia are closely linked to appetite suppression and progressive weight loss. Traditional treatments, such as appetite stimulants and nutritional support, have limited impact, highlighting the need for targeted therapies that address the underlying biological mechanisms.
Ponsegromab, a humanized monoclonal antibody, directly inhibits circulating GDF-15, blocking its interaction with GFRAL. This mechanism prevents anorexia and aids in preserving skeletal muscle, positioning Ponsegromab as a promising treatment for reversing the effects of cachexia.
Clinical Evidence: Ponsegromab in Action
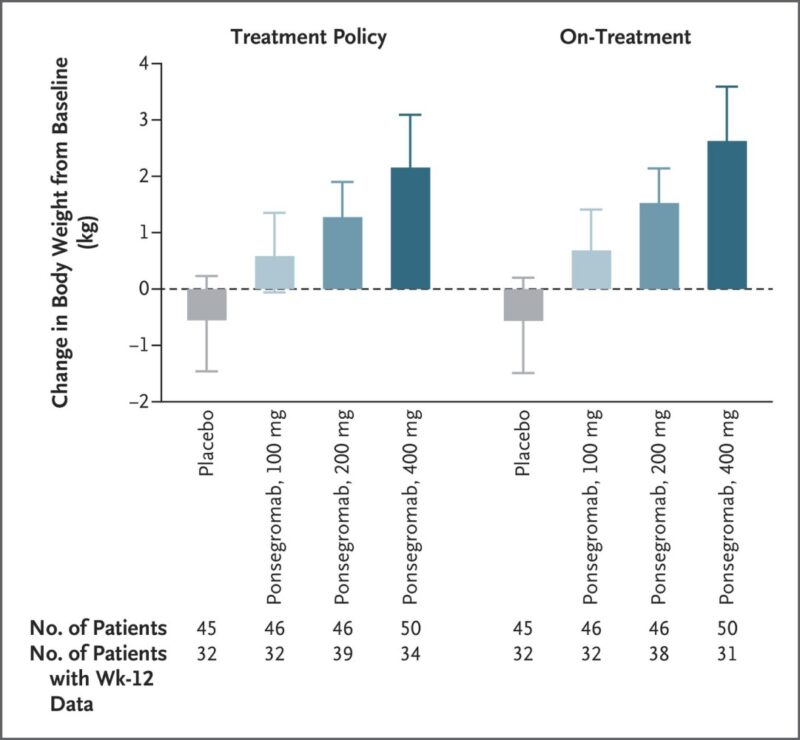
In a Phase II, randomized, double-blind study, 187 patients with advanced cancer and elevated GDF-15 levels (≥1500 pg/mL) were treated with 100 mg, 200 mg, or 400 mg of ponsegromab subcutaneously every 4 weeks for 12 weeks, or placebo. The study’s primary endpoint was the change in body weight at 12 weeks, while secondary endpoints included improvements in appetite, cachexia symptoms, physical activity, and safety.
Key findings from the trial were:
– Significant Weight Gain: Ponsegromab produced substantial weight gain across all dosing groups compared to placebo. At 12 weeks, patients in the 400 mg group gained a median of 2.81 kg compared to placebo, showing the most pronounced effect. Even those with severe weight loss experienced improvement, challenging the notion of refractory cachexia.
– Appetite and Physical Activity Improvement: Patients receiving 400 mg also reported marked improvements in appetite and reduced cachexia-related symptoms. Digital tracking of physical activity showed a 72-minute increase in non-sedentary activity per day, compared to placebo, indicating functional improvement.
– Safety Profile: Ponsegromab demonstrated a favorable safety profile, with fewer adverse events (such as nausea and vomiting) than placebo. Overall, 70% of patients on ponsegromab reported adverse events, compared to 80% in the placebo group. The placebo-like safety profile makes it suitable for use alongside other cancer therapies.
Implications for Clinical Practice
The findings from the Phase II trial suggest that ponsegromab may represent a significant breakthrough in the treatment of cancer cachexia, particularly for patients with elevated GDF-15 levels. By targeting a key driver of cachexia, ponsegromab offers more than just symptom management; it has the potential to reverse weight loss, enhance muscle mass, and improve patients’ ability to engage in daily activities.
These results are especially promising for patients with refractory cachexia, a population that has traditionally been difficult to treat. Ponsegromab’s ability to induce weight gain in these patients challenges long-standing assumptions about the irreversibility of advanced cachexia.
Further research is warranted to establish the optimal timing and dosing for initiating ponsegromab treatment in patients with cancer cachexia, but current evidence supports its potential role as a first-line therapy in managing this complex syndrome.
Conclusion
Ponsegromab represents a new frontier in the treatment of cancer cachexia, offering hope to patients and clinicians alike. By targeting the GDF-15 pathway, it provides a mechanism to prevent or reverse weight loss and improve physical function, with a safety profile conducive to long-term use. Future studies will clarify its role in the broader management of cachexia, but for now, ponsegromab stands out as a promising option in the fight against this life-limiting condition.
References:
- ESMO 2024 Congress, Ponsegromab Study Results.
- NEJM. Ponsegromab for Cancer Cachexia: A Phase II Randomized Controlled Trial.”
Akshaya Keerti is the International Council Deputy Director at the Federation of Medical Students Association (FMSA) and a Professional Member of Erevnites. He also serves as a Medical Officer at Meherbai Tata Memorial Cancer Hospital and is an Academic Lecturer with Project IMG.


