Paolo Tarantino, Advanced research fellow at Dana-Farber Cancer Institute, posted on X about recent paper by Xichun Hu et al., titled “ACE-Breast-02: a randomized phase III trial of ARX788 versus lapatinib plus capecitabine for HER2-positive advanced breast cancer” published on Signal Transduction and Targeted Therapy.
Authors: Xichun Hu, Qingyuan Zhang, Leiping Wang, Jian Zhang, Quchang Ouyang, Xiaojia Wang, Wei Li, Weimin Xie, Zhongsheng Tong, Shusen Wang, Faliang Xu, Tao Sun, Wei Liu, Zhendong Chen, Jinsheng Wu, Ying Wang, Haixia Wang, Min Yan, Xinshuai Wang, Jingfen Wang, Feilin Cao, Yingying Du, Yongqiang Zhang, Lilin Chen, Yuan Lei
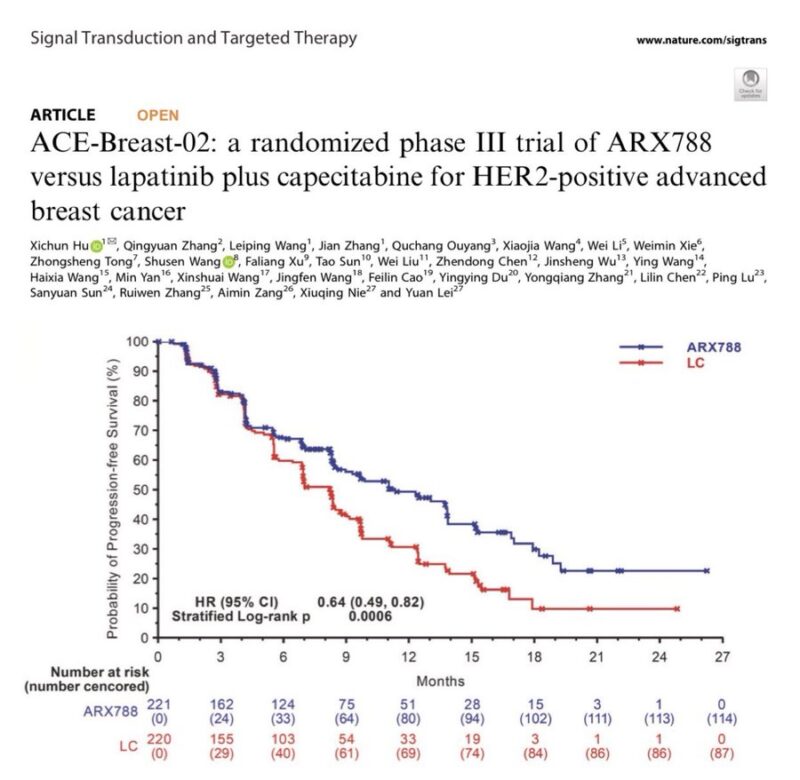
“ACE-Breast-02 is the first phase 3 trial testing a site-specific ADC. In AB-02, ARX788 improved PFS vs cape/lapatinib for HER2+ MBC, yet at the expense of high rate of ocular tox (>50%), hepatotoxicity (>70%) and ILD (>30%). ADC stability ≠ tolerability.”


