Mary E. Brunkow, Fred Ramsdell, and Shimon Sakaguchi recognized for discoveries in peripheral immune tolerance
Today, on 6 October 2025, the Nobel Assembly at the Karolinska Institutet announced that Mary E. Brunkow, Fred Ramsdell, and Shimon Sakaguchi will share the 2025 Nobel Prize in Physiology or Medicine
“for their discoveries concerning peripheral immune tolerance.”
Their work has illuminated how the immune system restrains itself beyond the central thymic checkpoint – insights with wide implications from autoimmunity to cancer.
These discoveries identify regulatory T cells (Tregs) and the master transcription factor FOXP3 as key to keeping immune responses in check. In effect, they discovered the molecular and cellular basis of how the immune system prevents collateral damage to self tissues.

What Is Peripheral Immune Tolerance – and Why It Was a Mystery
Historically, immunologists focused heavily on central tolerance: during T cell development in the thymus, autoreactive T cells that bind self-antigens too strongly are deleted. That model could not fully explain why many autoreactive clones still exist in the mature repertoire, but do not always cause disease.
Peripheral immune tolerance refers to the mechanisms that act outside the thymus (in the “periphery,” e.g. lymph nodes, tissues) to silence or restrain potentially harmful T cells. This includes mechanisms such as anergy, deletion, immune checkpoint signaling, and suppression by regulatory cells. The laureates’ core discovery was that a subset of T cells is dedicated to actively suppressing autoimmunity: these are the regulatory T cells (Tregs).
Their further breakthrough was finding that the gene FOXP3 is essential for generating functional Tregs. Mutations in this gene in mice lead to a fatal autoimmune “scurfy” phenotype; in humans, mutations cause IPEX syndrome (immune dysregulation, polyendocrinopathy, enteropathy). Thus, the field moved from conceptual suppression to a defined molecular axis of tolerance.
Milestones: From Hypothesis to Molecular Mechanism
Sakaguchi’s paradigm shift
In 1995, Shimon Sakaguchi challenged the notion that deletion in the thymus was sufficient. He observed that neonatal thymectomy (removal of the thymus) induced autoimmunity, but could be prevented by transfer of mature T cells. This led him to propose and then isolate the suppressive T cell subset (Tregs) that exist in the periphery. Over years, his lab characterized these cells, their phenotypes, and functional roles.
Brunkow & Ramsdell – the FOXP3 story
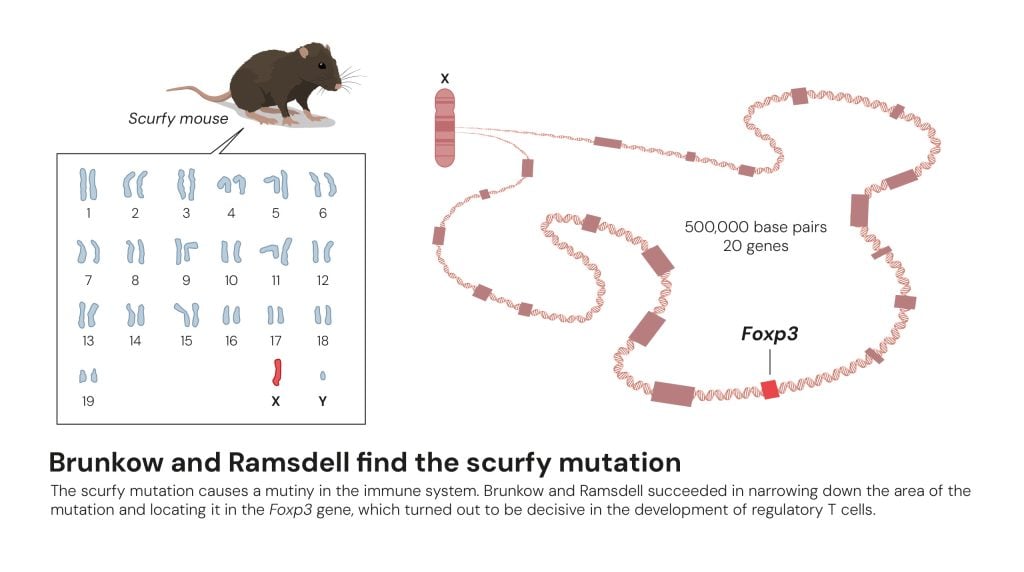
Around 2001, Mary E. Brunkow and Fred Ramsdell homed in on a genetic mutant mouse, called scurfy, whose lethal immunopathology stemmed from a defect in immune regulation. They mapped the mutation to the X chromosome and discovered it disrupted a forkhead transcription factor gene, later designated FOXP3. They then extended their mouse findings to humans by linking FOXP3 mutations to the IPEX syndrome.
Interactions among the labs soon converged: Sakaguchi’s Tregs turned out to depend on FOXP3, making a coherent model for peripheral immune tolerance.
The Laureates: Stories and Scientific Journeys
Mary E. Brunkow – The Genetic Detective Who Found the Missing Link
For Mary E. Brunkow, discovery began with a mystery mouse. Born in 1961, she earned her Ph.D. in Molecular Biology from Princeton University, driven by an early fascination with how single genes control complex immune behaviors. After her studies, she joined Celltech in the UK, a bold move that immersed her in the fast-growing world of biotech research.

Her breakthrough came while studying the “scurfy” mouse, a mutant plagued by lethal autoimmunity. Brunkow traced its disease to a single gene on the X chromosome – FOXP3 – and revealed that this transcription factor governs the development and function of regulatory T cells (Tregs). This finding linked a rare animal model to a human syndrome, IPEX, forever changing how scientists understood immune self-tolerance.
Now at the Institute for Systems Biology in Seattle, Brunkow continues to champion the integration of genetics and systems immunology. Her precision in decoding genetic defects has given immunology one of its most fundamental tools—and a new vocabulary for how balance is maintained within the immune system.
Mary E. Brunkow’s First Reaction after learning about he won the Nobel Prize in Physiology or Medicine 2025 | Telephone interview
Fred Ramsdell – The Architect of Translation
Fred Ramsdell earned his Ph.D. in Immunology from UCLA and has built a career that seamlessly connects discovery and application. With experience at Amgen, Zymogenetics, and now Sonoma Biotherapeutics, he has helped steer the transition of immune tolerance research from academic insight to clinical innovation.

Working alongside Brunkow, Ramsdell was pivotal in identifying FOXP3 as the missing gene in the scurfy mouse and showing that its human counterpart causes IPEX syndrome. But his vision extended beyond discovery, he recognized early that these insights could lead to cell-based immune therapies capable of restoring tolerance in autoimmune and transplant settings.
Ramsdell’s path reflects the modern scientist-builder: part immunologist, part strategist. His influence lies not only in the lab bench breakthroughs but in shaping the biotech ecosystem that now seeks to harness Tregs as living drugs.
Shimon Sakaguchi – The Visionary Who Saw the Invisible
Decades before FOXP3 entered the story, Shimon Sakaguchi was asking how the immune system knows when to stop attacking. Born in Shiga Prefecture, Japan, in 1951, and trained at Kyoto University, he followed his instincts into a field few dared to explore, the biology of immune suppression.

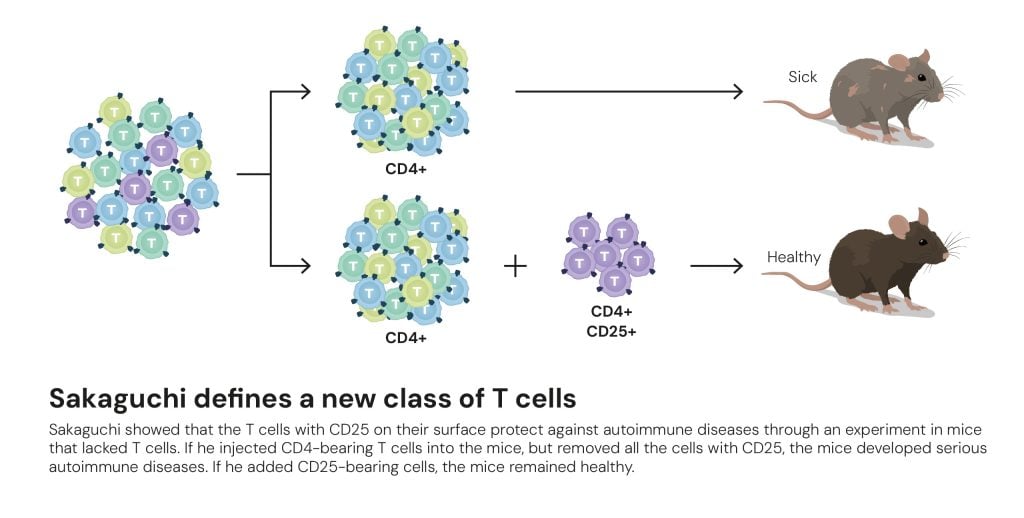
In the mid-1990s, Sakaguchi made a discovery that changed immunology forever: when a small subset of T cells marked by CD25 was removed from mice, they developed severe autoimmune disease. These cells, he showed, acted as natural suppressors of immune activation, what he named regulatory T cells.
His persistence through skepticism defined him as both experimentalist and visionary. By the early 2000s, his work intersected with the FOXP3 discoveries, completing the picture of peripheral immune tolerance. As a professor at Osaka University, Sakaguchi continues to guide global immunology through his pioneering studies of Tregs in infection, autoimmunity, and cancer.
Shimon Sakaguchi’s First Reaction after learning about he won the Nobel Prize in Physiology or Medicine 2025 | Telephone interview
Together: A New Map of Immunity
Mary Brunkow uncovered the genetic blueprint, Fred Ramsdell built the bridge to therapy, and Shimon Sakaguchi revealed the hidden guardians of self-tolerance. Together, they transformed the concept of immune restraint from speculation into molecular science.
Their discoveries have become the foundation for new generations of treatments, from autoimmune repair to fine-tuned cancer immunotherapy, reminding us that true progress lies not only in attacking disease, but in understanding how the body chooses peace.

Fred Ramsdell, Mary E. Brunkow and Shimon Sakaguchi
Nobel Prize: Significance, Prestige, and Place in the Biomedical Ecosystem
The Nobel Prize is among the most prestigious and globally recognized honors in science. Established by Alfred Nobel’s will in the late 19th century, it awards major breakthroughs in physics, chemistry, physiology or medicine, literature, peace, and (later) economics. The Medicine/Physiology prize has long served as a marker of paradigm-shifting discoveries that deeply influence human health and biology.

Winning a Nobel often accelerates funding, attracts global attention, and cements a discovery’s place in textbooks. It also signals to the scientific and medical community which areas are considered foundational – or on the cusp of translation.
In the realm of oncology, many Nobel laureates have laid the foundations of immunotherapy, targeted therapy, tumor biology, or translational cancer mechanisms. For example, the 2018 Nobel Prize went to James P. Allison and Tasuku Honjo for unveiling immune checkpoint brakes (CTLA-4, PD-1) and enabling checkpoint blockade in cancer therapy.

The 2025 laureates’ discovery of immune regulation adds a complementary dimension: understanding not only how to release immune brakes (for cancer) but also how the system is intrinsically constrained, knowledge that may help refine the safety and precision of cancer immunotherapies.
Thus, this award is particularly relevant to oncologists and cancer researchers: it underscores the double-edged nature of immune control and invites new design of therapies that modulate, rather than override, regulatory circuits.
Closing Thoughts
The Nobel Prize 2025 in Medicine highlights a milestone in immunology: the unveiling of how the immune system restrains itself, not just how it activates. Brunkow, Ramsdell, and Sakaguchi have transformed abstract ideas about “immune suppression” into a mechanistic and molecular framework, revealing the intimate balance between attack and restraint in immunity.
For cancer and immunology communities, these insights invite more nuanced strategies: how to disable Tregs in tumors temporarily, how to reprogram them in autoimmunity, and how to finetune this balance in transplantation and beyond. As research and clinical trials now rush forward, the laureates’ legacy will likely catalyze novel therapies across diseases.
Learn more:
OncoDaily has previously covered Nobel Prize news and its impact on biomedical science.
You can revisit our coverage on
Victor Ambros and Gary Ruvkun as the 2024 Nobel Medicine laureates
for microRNA discoveries in our OncoDaily archive.
2024 Nobel Prize in Chemistry: Advancements in Protein Design and Structure Prediction
Written by Sergey Badalyan, MD