The NEST Trial, conducted by Agenus, was presented with latest updates at the ASCO GI 2025 Symposium, showcasing the use of Botensilimab in combination with Balstilimab as neoadjuvant immunotherapy with NO RECURRENCE in MSS Colorectal Cancer for patients who were candidates for surgery.
A detailed comment on the NEST Trial was shared by the PI of the study, Pashtoon Kasi, Medical Director of GI Medical Oncology at City of Hope, on his recent post on X:
“No recurrences!
Neoadjuvant immunotherapy keeps delivering! Patients with MSS, not just MSI-High colorectal cancer.
Goal is to cure more patients. Phase2/3
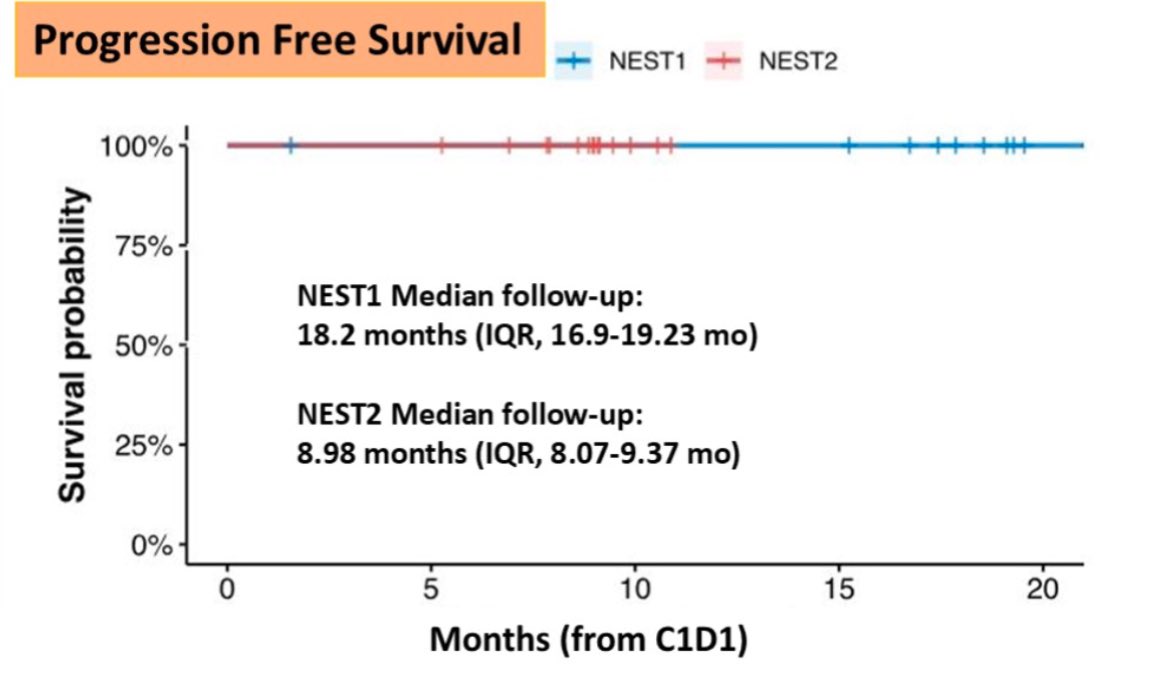
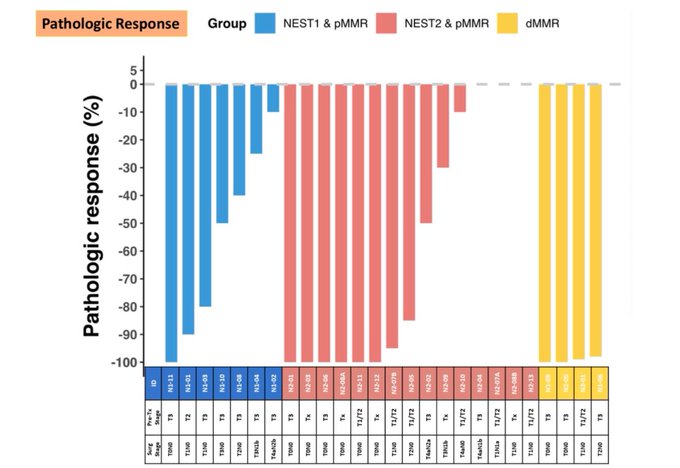
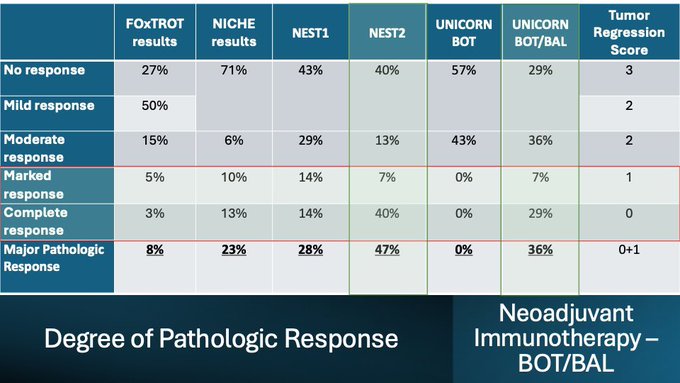
We also helped answer an important question. Do responses deepen by letting immunotherapy brew for longer. You can visually see surgery timing: 4 weeks VS 8 weeks, the proportion of nearCR + pathCR (major pathologic responses).
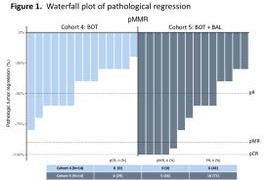
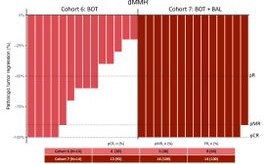
The UNICORN study of BOT+/-BAL corroborates the findings of our NEST trial. Also provides:
“Contribution of Components”.
BOT needs its partner BAL.
Not entirely surprising since earlier data on IPI/NIVO also showed that CTLA4 was not sufficient.
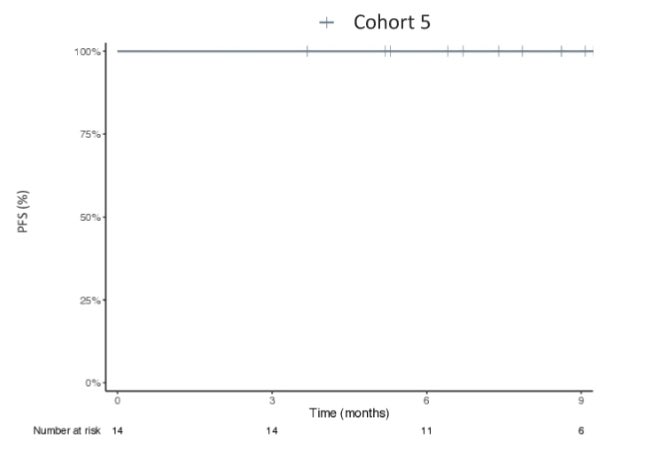
Here’s the replica of the flatline from the UNICORN study, as noted in NEST trial as well.
From BOT+BAL arm for patients with MSS colorectal cancer.
On the question of adjuvant chemotherapy; for this NEST trial & other trials, it’s best to let the treating physician/patient decide.
A lot of variability.
3 months vs 6 months , doublet vs single, FOLFOX vs CAPEOX. It was recommended but not uncommon for it to be refused.
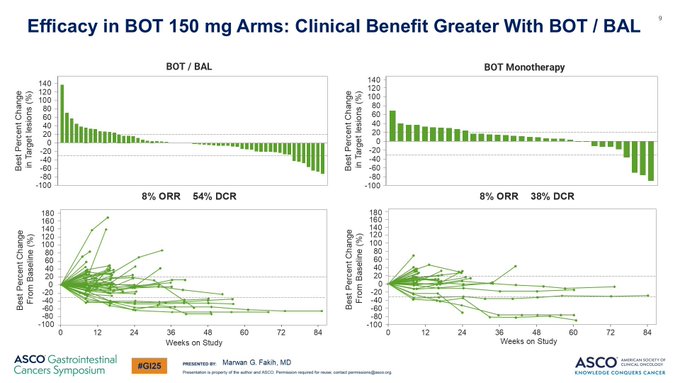
More on contribution of components & also the activity in the setting in which you use immunotherapy.
Data just being presented by Dr Marwan G. Fakih shows not only the great, durable response vs standard of care, but also that you need the PD-1 alongside CTLA4 BOT.
Adding the toxicity question here again.
Between the
- Global study
- NEST &
- UNICORN
We know:
- setting matters (NLM & Neoadjuvant)
- dosing
- contribution of components (BOT+BAL)
an option for our patients.
It’s not on the agents we use, but also the amount of time we give it safely to work before surgery occurs.
Most important message is that adopting the NEOADJUVANT setting paradigm change as a platform for immunotherapy & drug development is safe/feasible.”
About the NEST Trial
The NEST trial conducted by Agenus Inc., aims to explore the safety and efficiency of neoadjuvant botensilimab (BOT), an Fc-enhanced next-generation anti–CTLA–4 antibody, alongside balstilimab (BAL; an anti-PD-1 antibody) in patients with colon and rectal cancer who were candidates for surgery.
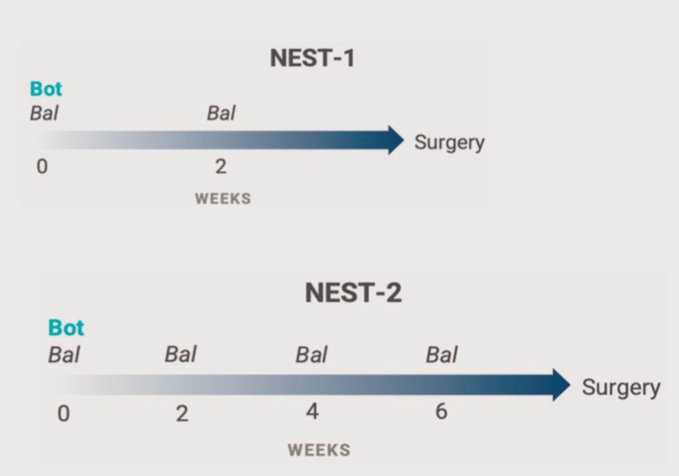
It is a single arm trial of neoadjuvant BOT 75 mg/m2 (day 1) and BAL 240 mg/m2 every 2 weeks x2 (NEST1-NCT05571293) or x4 (NEST2-NCT05571293).
NEST2 has shown increased response rates compared to NEST1, potentially due to additional doses of balstilimab and a longer interval before surgery.
About Botensilimab
Botensilimab is a human Fc enhanced CTLA-4 blocking antibody designed to boost both innate and adaptive anti-tumor immune responses. Its novel design leverages mechanisms of action to extend immunotherapy benefits to “cold” tumors which generally respond poorly to standard of care or are refractory to conventional PD-1/CTLA-4 therapies and investigational therapies. Botensilimab augments immune responses across a wide range of tumor types by priming and activating T cells, downregulating intratumoral regulatory T cells, activating myeloid cells and inducing long-term memory responses.
Botensilimab is one of the drugs featured in OncoDaily’s “10 Most Promising Cancer Drugs Not Yet Approved: Solid Tumors – 2024 edition by OncoDaily.”
Approximately 1100 patients have been treated with botensilimab across various clinical trial phases, yielding promising results in nine different metastatic cancers. Botensilimab, either alone or combined with Agenus’ experimental PD-1 antibody balstilimab, has demonstrated clinical responses in nine different metastatic, late-stage cancers.
In the inaugural event of the Global Cancer Movement: Challenging the Status Quo in Colorectal Cancer, initiated by OncoDaily, Dr. Pashtoon Kasi delves into pioneering advancements in colorectal cancer treatment.
“The notion of immunotherapy does not work for colon cancer is an incorrect statement. Moving forward, I would say, commercially available immunotherapy does not work for colorectal cancer, but with novel agents like botensilimab and other c-enhanced drugs looking into the space, it’s promising to see the activity of immunotherapy now making its way for patients with colorectal cancer. Because targeted therapies, they are all excited things, but I think if there is one type of therapy where cures lie or where I started hearing the word cure, it was all immunotherapy responders.“– said Dr. Kasi.
About Agenus
Agenus Inc. is a clinical-stage immuno-oncology company focused on the discovery and development of therapies that engage the body’s immune system to fight cancer. Founded in 1994 and headquartered in Lexington, Massachusetts, Agenus has built a diverse pipeline of immune-modulating antibodies, cancer vaccines, and adoptive cell therapies.
The company’s approach to cancer immunotherapy is multifaceted, encompassing checkpoint inhibitors, bispecific antibodies, cell therapies, cancer vaccines, and adjuvants.
Further readings:
- Botensilimab: A Revolutionary Fc-Enhanced CTLA-4 Inhibitor Transforming Cancer Immunotherapy
- Three decades of pioneering in Immune Oncology – Agenus
- Agenus announced new data highlighting BOT/BAL across multiple lines of treatment in Colorectal Cancer
- Immunotherapy for Colon Cancer: Types, Success Rate, Side Effects & More