Hanna Foster, Scientific Product Sales Manager at Quadratech DiagnosticsLtd, shared a post on LinkedIn:
“Integrating MRD testing into clinical care for patients with solid tumours… One indication at a time.
MRD (minimal/ molecular/ measurable residual disease) describes persistence of small number of cancer cells in the body after treatment. MRD detection requires advanced technologies capable of detecting low levels of circulating tumour DNA (ctDNA) even when cancer is undetectable using traditional imaging or standard tests.
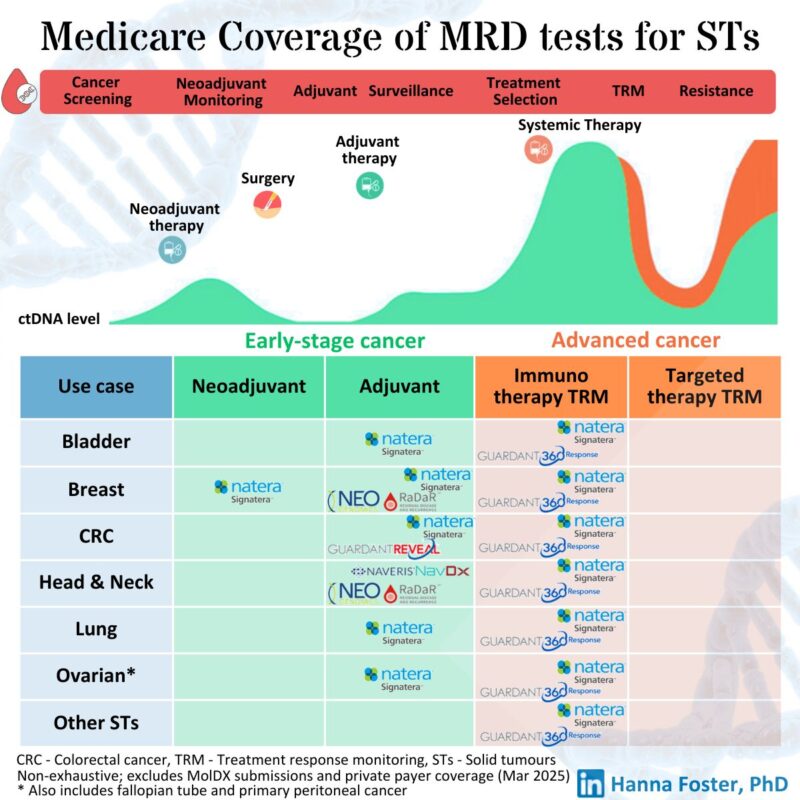
MRD tests can be used to personalise and guide clinical cancer care (aka crystal ball to tell if cancer is coming back, treatment is working or may no longer be needed – all clear)
- Monitoring response to neoadjuvant therapy (before surgery)
- MRD-based stratification to guide adjuvant (post-surgery) therapy
- Adjuvant surveillance for treatment escalation and de-escalation
- Monitoring response to systemic therapy in patients with advanced cancers
Despite all the potential clinical value, MRD testing is yet to become routine in clinic, especially for patients with solid tumours.
While 18 million cancer patients in the US could benefit from MRD testing, less than 3% are being tested, likely due to:
– Clinical evidence generation lagging behind technology advancements
– Need for benchmarking and standardisation of MRD tests
– Technical limits of detection for low-shedding tumours
– Lack of clear guideline recommendations on clinical value
– Restricted public and private reimbursement by:
- Cancer indication and subtype (NavDx for HPV+ vs. RaDaR for HPV- head and neck cancer)
- Cancer stage (Early operable vs. advanced metastatic)
- Setting (Neoadjuvant vs. adjuvant)
- Coverage (Early post-op vs. surveillance, single test vs. bundle)
- Recommended testing interval
- Therapy class
But the tide is turning and the clinical adoption of MRD tests is gaining momentum, at least in the US. Clinical volumes of Natera’s Signatera jumped up to ~500 K in 2024 (~64% YoY).
2025 already saw:
- NCCN strengthening guidelines on ctDNA in colon and rectal cancer (prognostic marker) and merkel cell carcinoma (positive recommendation for surveillance)
- Medicare coverage for Natera’s Signatera in new indications, with the latest being early-stage NSCLC
- Expanded Medicare coverage for Guardant Reveal in CRC, now including surveillance
- Multiple submissions and peer-reviewed publications of clinical validation data for MRD tests (a prerequisite for reimbursement)
What’s next
- Medicare and commercial coverage in additional indications and settings. Which one will be next?
- First Medicare reimbursement for new entrants (incl. Personalis NeXT Personal Dx)
- Updates on IP litigations and injunctions (incl. NeoGenomics’ RaDaR)
Inclusion of MRD/ ctDNA in NCCN clinical practice guidelines for CRC. Or is it a wishful thinking?
Broader clinical adoption of MRD tests for solid tumours in the US. When will Europe and RoW follow?”
Sucharu Prakash, Director of Quality Services at Texas Oncology, shared this post on LinkedIn, adding a comment:
“Great post. MRD IN SOLID TUMORS NOT READY FOR PRIMETIME! WE NEED MORE DATA!
Molecular residual disease (MRD) is an intuitive concept- detect ctDNA and find cancer recurrence early-However, MRD testing is not endorsed by NCCN or ASCO guidelines.
Why?- because these assays have not been shown to have “clinical utility” and numerous reports show NO improvement in survival!
We need more RESEARCH! Many labs now offer MRD testing but best done as part of a study.
What about payor coverage ?– getting better thanks to Biomarker bill SB 989 which we lobbied for.
I continue to stress NGS testing for therapeutic decision making but will wait to recommend universal MRD testing”
More posts featuring Hanna Foster and Sucharu Prakash on OncoDaily.
Further Reading:
New Paper Alert: ctDNA Clearance Predicts pCR in Solid Tumors Treated with Neoadjuvant Immunotherapy


