Residual breast cancer after neoadjuvant chemotherapy remains a major challenge, with high recurrence risks and limited effective post-neoadjuvant options. A new study published in Clinical Breast Cancer analyzes data from the DFCI 05-055 and 09-134 clinical trials, evaluating the role of bevacizumab-based therapy and capecitabine in improving outcomes. While bevacizumab showed no significant survival benefit, capecitabine demonstrated improved recurrence-free survival (RFS) in triple-negative breast cancer (TNBC). These findings reinforce the need for personalized, biomarker-driven treatment strategies to optimize therapy for high-risk patients.
Title: Optimizing Postneoadjuvant Treatment of Residual Breast Cancer With Adjuvant Bevacizumab Alone, With Metronomic or Standard-Dose Chemotherapy: A Combined Analysis of DFCI 05-055 and DFCI 09-134/TBCRC 012/ABCDE Clinical Trials
Published in Clinical Breast Cancer, in January 2025
Authors : Dario Trapani , Qingchun Jin, Kathy D. Miller, Hope S. Rugo, Katherine E. Reeder-Hayes, Tiffany Traina, Yara Abdou, Carla Falkson, Vandana Abramson, Jennifer Ligibel, Wendy Chen, Steven Come, Anju Nohria, Nicole Ryabin, Nabihah Tayob, Sara M Tolaney , Harold J. Burstein, Erica L. Mayer
Background
Patients with early-stage breast cancer who have residual breast cancer following neoadjuvant chemotherapy are at an increased risk of recurrence and poorer survival compared to those who achieve a pathologic complete response (pCR). These high-risk patients often require additional systemic therapy in the adjuvant setting to reduce recurrence risk.
Bevacizumab, an anti-VEGF monoclonal antibody, has been investigated in various settings to improve survival by inhibiting angiogenesis. While it has demonstrated improved pCR rates in the neoadjuvant setting, its long-term survival benefits in early-stage breast cancer remain uncertain. This combined analysis evaluates two independent trials (05-055 and 09-134) that assessed the feasibility and efficacy of bevacizumab-based post-neoadjuvant therapy in patients with residual breast cancer after neoadjuvant chemotherapy.
Methods & Study Design
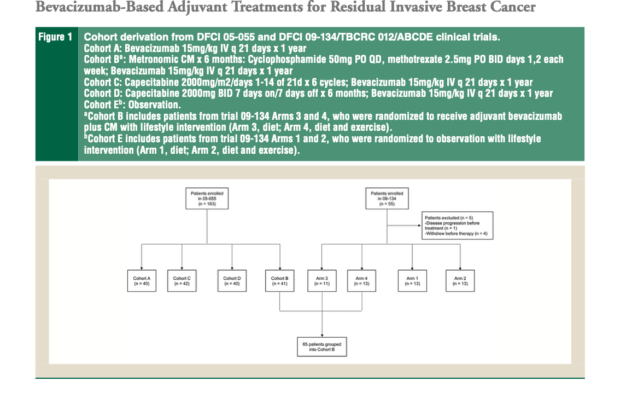
This study analyzed data from two clinical trials evaluating different bevacizumab-containing post-neoadjuvant regimens in early-stage breast cancer. The study population included patients with stage I-III breast cancer who had residual disease following neoadjuvant chemotherapy. Treatment regimens in the trials included:
- Bevacizumab monotherapy
- Bevacizumab with metronomic cyclophosphamide/methotrexate (CM)
- Bevacizumab with flat-dose capecitabine
- Bevacizumab with body surface area (BSA)-dosed capecitabine
The primary endpoints included recurrence-free survival (RFS) at 36 months and treatment feasibility based on toxicity and discontinuation rates. Secondary endpoints included overall survival (OS) and biomarker analysis for plasma thrombospondin-1 (TSP-1) to evaluate angiogenesis inhibition.
Results
Recurrence-Free Survival (RFS)
- Bevacizumab monotherapy: 58.0%
- Bevacizumab + metronomic CM: 62.3%
- Bevacizumab + flat-dose capecitabine: 72.7%
- Bevacizumab + BSA-based capecitabine: 75.0%
Although bevacizumab with capecitabine showed a numerical improvement in RFS, the results were not statistically significant compared to observation.
- Grade 3-4 adverse events (AEs) were most frequent in:
- Bevacizumab + flat-dose capecitabine (72.5%)
- Bevacizumab + BSA-dosed capecitabine (71.4%)
- BSA-dosed capecitabine resulted in higher treatment discontinuation rates (31%) compared to flat-dose capecitabine (12.5%).
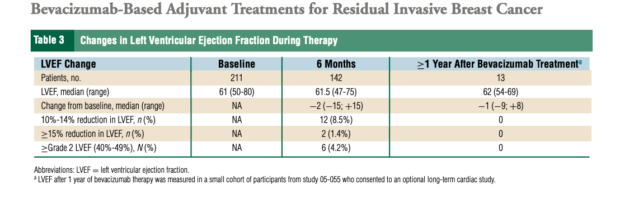
- Bevacizumab-related toxicities, including hypertension, proteinuria, and bleeding, were consistent with prior studies.
Triple-Negative Breast Cancer (TNBC) Outcomes
Among patients with TNBC, capecitabine was associated with significantly improved RFS (HR: 0.47; 95% CI, 0.23-0.96), reinforcing its benefit as seen in the CREATE-X trial, which demonstrated improved disease-free survival (DFS) and OS with capecitabine in patients with residual invasive disease.
Biomarker Analysis
TSP-1 plasma levels were analyzed to assess angiogenesis inhibition. The results did not suggest a strong antiangiogenic effect of bevacizumab-based therapy in the post-neoadjuvant setting. The absence of a comprehensive angiogenic biomarker panel limits conclusions regarding bevacizumab’s mechanistic impact.
Key Findings
- Bevacizumab did not significantly improve survival in the post-neoadjuvant setting for residual breast cancer, aligning with prior studies such as CALGB 40603 and E5103, which also found no long-term benefit with bevacizumab in early-stage breast cancer.
- Capecitabine was associated with improved RFS in TNBC, further supporting its role in post-neoadjuvant therapy.
- Flat-dose capecitabine was better tolerated than BSA-dosed capecitabine, suggesting a potential preference for fixed dosing in future trials.
- Angiogenesis inhibition, as assessed by TSP-1, was not robust, raising questions about the role of bevacizumab in this setting.
Key Takeaway Messages
- Bevacizumab-based post-neoadjuvant therapy is feasible but does not improve survival outcomes in early-stage breast cancer.
- Standard-dose capecitabine remains an effective post-neoadjuvant option for TNBC.
- Patients with high-risk disease may benefit from newer strategies, including immune checkpoint inhibitors, PARP inhibitors, and CDK4/6 inhibitors.
- Risk-adapted strategies and circulating tumor DNA (ctDNA)-based minimal residual disease (MRD) detection could help optimize adjuvant therapy.
Comparison to Other Trials & Emerging Therapies
Several trials have assessed newer post-neoadjuvant strategies for high-risk early-stage breast cancer:
- KEYNOTE-522: Pembrolizumab in neoadjuvant and adjuvant settings improved event-free survival (HR: 0.63; P < .001) and OS in TNBC.
- A-BRAVE: Adjuvant avelumab improved OS but not DFS in early-stage TNBC.
- IMpassion030: Atezolizumab added to adjuvant chemotherapy did not improve DFS.
- OlympiA: Olaparib improved iDFS (HR: 0.58; P < .001) and OS in BRCA-mutated HER2-negative breast cancer.
- monarchE & NATALEE: CDK4/6 inhibitors abemaciclib and ribociclib improved iDFS in HR+/HER2-negative disease.
- KATHERINE: T-DM1 was superior to trastuzumab in HER2+ disease with residual disease after neoadjuvant therapy (HR: 0.50; P < .001).
Limitations
- Patients were treated between 2005-2013, before the introduction of immune checkpoint inhibitors and targeted therapies.
- The 09-134 trial did not complete accrual, limiting statistical power.
- The comparison across treatment arms was non-randomized, potentially introducing bias.
- Survival follow-up was limited, particularly for HR+ breast cancer.
Conclusion
The combined analysis of trials 05-055 and 09-134 found that adjuvant bevacizumab, with or without chemotherapy, was feasible but did not improve survival outcomes. In contrast, standard-dose capecitabine showed improved RFS in TNBC, reinforcing its role in post-neoadjuvant treatment. Given the high recurrence risk in patients with residual disease, newer approaches such as immune checkpoint inhibitors, PARP inhibitors, and CDK4/6 inhibitors should be prioritized in high-risk populations. Future research should focus on biomarker-driven treatment adaptation, MRD-based therapy modulation, and personalized post-neoadjuvant strategies to optimize outcomes in early-stage breast cancer.
Read Full Article Here